Carbohydrate- and conformation-dependent cargo capture for ER-exit
- PMID: 15635097
- PMCID: PMC551490
- DOI: 10.1091/mbc.e04-08-0708
Carbohydrate- and conformation-dependent cargo capture for ER-exit
Abstract
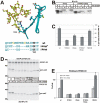
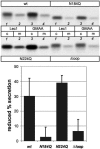
Some secretory proteins leave the endoplasmic reticulum (ER) by a receptor-mediated cargo capture mechanism, but the signals required for the cargo-receptor interaction are largely unknown. Here, we describe a novel targeting motif that is composed of a high-mannose type oligosaccharide intimately associated with a surface-exposed peptide beta-hairpin loop. The motif accounts for lectin ERGIC-53-assisted ER-export of the lyososomal enzyme procathepsin Z. The second oligosaccharide chain of procathepsin Z exhibits no binding activity for ERGIC-53, illustrating the selective lectin properties of ERGIC-53. Our data suggest that the conformation-based motif is only present in fully folded procathepsin Z and that its recognition by ERGIC-53 reflects a quality control mechanism that acts complementary to the primary folding machinery in the ER. A similar oligosaccharide/beta-hairpin loop structure is present in cathepsin C, another cargo of ERGIC-53, suggesting the general nature of this ER-exit signal. To our knowledge this is the first documentation of an ER-exit signal in soluble cargo in conjunction with its decoding by a transport receptor.
Figures





Similar articles
-
pH-induced conversion of the transport lectin ERGIC-53 triggers glycoprotein release.J Biol Chem. 2004 Mar 26;279(13):12943-50. doi: 10.1074/jbc.M313245200. Epub 2004 Jan 12. J Biol Chem. 2004. PMID: 14718532
-
The lectin ERGIC-53 is a cargo transport receptor for glycoproteins.Nat Cell Biol. 1999 Oct;1(6):330-4. doi: 10.1038/14020. Nat Cell Biol. 1999. PMID: 10559958
-
Structures of the carbohydrate recognition domain of Ca2+-independent cargo receptors Emp46p and Emp47p.J Biol Chem. 2006 Apr 14;281(15):10410-9. doi: 10.1074/jbc.M512258200. Epub 2006 Jan 26. J Biol Chem. 2006. PMID: 16439369
-
Lectins and protein traffic early in the secretory pathway.Biochem Soc Symp. 2002;(69):73-82. doi: 10.1042/bss0690073. Biochem Soc Symp. 2002. PMID: 12655775 Review.
-
Role of N-oligosaccharide endoplasmic reticulum processing reactions in glycoprotein folding and degradation.Biochem J. 2000 May 15;348 Pt 1(Pt 1):1-13. Biochem J. 2000. PMID: 10794707 Free PMC article. Review.
Cited by
-
Identification of GOLPH3 Partners in Drosophila Unveils Potential Novel Roles in Tumorigenesis and Neural Disorders.Cells. 2021 Sep 6;10(9):2336. doi: 10.3390/cells10092336. Cells. 2021. PMID: 34571985 Free PMC article.
-
IER3IP1-mutations cause microcephaly by selective inhibition of ER-Golgi transport.Cell Mol Life Sci. 2024 Aug 8;81(1):334. doi: 10.1007/s00018-024-05386-x. Cell Mol Life Sci. 2024. PMID: 39115595 Free PMC article.
-
A cyclooxygenase-2-dependent prostaglandin E2 biosynthetic system in the Golgi apparatus.J Biol Chem. 2015 Feb 27;290(9):5606-20. doi: 10.1074/jbc.M114.632463. Epub 2014 Dec 29. J Biol Chem. 2015. PMID: 25548276 Free PMC article.
-
Intracellular lectins are involved in quality control of glycoproteins.Proc Jpn Acad Ser B Phys Biol Sci. 2014;90(2):67-82. doi: 10.2183/pjab.90.67. Proc Jpn Acad Ser B Phys Biol Sci. 2014. PMID: 24522156 Free PMC article. Review.
-
A luminal flavoprotein in endoplasmic reticulum-associated degradation.Proc Natl Acad Sci U S A. 2009 Sep 1;106(35):14831-6. doi: 10.1073/pnas.0900742106. Epub 2009 Aug 19. Proc Natl Acad Sci U S A. 2009. PMID: 19706418 Free PMC article.
References
-
- Appenzeller, C., Andersson, H., Kappeler, F., and Hauri, H. P. (1999). The lectin ERGIC-53 is a cargo transport receptor for glycoproteins. Nat. Cell Biol. 1, 330-334. - PubMed
-
- Appenzeller-Herzog, C., Roche, A. C., Nufer, O., and Hauri, H. P. (2004). pH-induced conversion of the transport lectin ERGIC-53 triggers glycoprotein release. J. Biol. Chem. 279, 12943-12950. - PubMed
-
- Barlowe, C. (2003). Signals for COPII-dependent export from the ER: what's the ticket out? Trends Cell Biol. 13, 295-300. - PubMed
-
- Belden, W. J., and Barlowe, C. (2001). Role of Erv29p in collecting soluble secretory proteins into ER-derived transport vesicles. Science 294, 1528-1531. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

