A plasma membrane pool of phosphatidylinositol 4-phosphate is generated by phosphatidylinositol 4-kinase type-III alpha: studies with the PH domains of the oxysterol binding protein and FAPP1
- PMID: 15635101
- PMCID: PMC551492
- DOI: 10.1091/mbc.e04-07-0578
A plasma membrane pool of phosphatidylinositol 4-phosphate is generated by phosphatidylinositol 4-kinase type-III alpha: studies with the PH domains of the oxysterol binding protein and FAPP1
Abstract
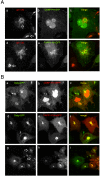
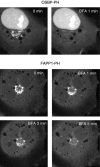
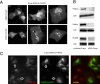
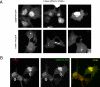
The PH domains of OSBP and FAPP1 fused to GFP were used to monitor PI(4)P distribution in COS-7 cells during manipulations of PI 4-kinase (PI4K) activities. Both domains were associated with the Golgi and small cytoplasmic vesicles, and a small fraction of OSBP-PH was found at the plasma membrane (PM). Inhibition of type-III PI4Ks with 10 microM wortmannin (Wm) significantly reduced but did not abolish Golgi localization of either PH domains. Downregulation of PI4KIIalpha or PI4KIIIbeta by siRNA reduced the localization of the PH domains to the Golgi and in the former case any remaining Golgi localization was eliminated by Wm treatment. PLC activation by Ca2+ ionophores dissociated the domains from all membranes, but after Ca2+ chelation, they rapidly reassociated with the Golgi, the intracellular vesicles and with the PM. PM association of the domains was significantly higher after the Ca2+ transient and was abolished by Wm pretreatment. PM relocalization was not affected by down-regulation of PI4KIIIbeta or -IIalpha, but was inhibited by down-regulation of PI4KIIIalpha, or by 10 microM PAO, which also inhibits PI4KIIIalpha. Our data suggest that these PH domains detect PI(4)P formation in extra-Golgi compartments under dynamic conditions and that various PI4Ks regulate PI(4)P synthesis in distinct cellular compartments.
Figures






References
-
- Audhya, A., and Emr, S. D. (2002). Stt4 PI 4-kinase localizes to the plasma membrane and functions in the Pkc1-mediated MAP kinase cascade. Dev. Cell 2, 593-605. - PubMed
-
- Balla, A., Tuymetova, G., Barshishat, M., Geiszt, M., and Balla, T. (2002). Characterization of type II phosphatidylinositol 4-kinase isoforms reveals association of the enzymes with endosomal vesicular compartments. J. Biol. Chem. 277, 20041-20050. - PubMed
-
- Balla, T., Bondeva, T., and Varnai, P. (2000). How accurately can we image inositol lipids in live cells? Trends Pharmacol. Sci. 21, 238-241. - PubMed
-
- Balla, T. and Varnai, P. (2002). Visualizing cellular phosphoinositide pools with GFP-fused protein-modules. Sci. STKE 125, PL3, 1-16. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

