Positive co-operative activity and dimerization of the isolated ABC ATPase domain of HlyB from Escherichia coli
- PMID: 15636583
- PMCID: PMC1134867
- DOI: 10.1042/BJ20041282
Positive co-operative activity and dimerization of the isolated ABC ATPase domain of HlyB from Escherichia coli
Abstract
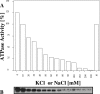
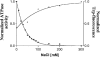
The ATPase activity of the ABC (ATP-binding cassette) ATPase domain of the HlyB (haemolysin B) transporter is required for secretion of Escherichia coli haemolysin via the type I pathway. Although ABC transporters are generally presumed to function as dimers, the precise role of dimerization remains unclear. In the present study, we have analysed the HlyB ABC domain, purified separately from the membrane domain, with respect to its activity and capacity to form physically detectable dimers. The ATPase activity of the isolated ABC domain clearly demonstrated positive co-operativity, with a Hill coefficient of 1.7. Furthermore, the activity is (reversibly) inhibited by salt concentrations in the physiological range accompanied by proportionately decreased binding of 8-azido-ATP. Inhibition of activity with increasing salt concentration resulted in a change in flexibility as detected by intrinsic tryptophan fluorescence. Finally, ATPase activity was sensitive towards orthovanadate, with an IC50 of 16 microM, consistent with the presence of transient dimers during ATP hydrolysis. Nevertheless, over a wide range of protein or of NaCl or KCl concentrations, the ABC ATPase was only detected as a monomer, as measured by ultracentrifugation or gel filtration. In contrast, in the absence of salt, the sedimentation velocity determined by analytical ultracentrifugation suggested a rapid equilibrium between monomers and dimers. Small amounts of dimers, but apparently only when stabilized by 8-azido-ATP, were also detected by gel filtration, even in the presence of salt. These data are consistent with the fact that monomers can interact at least transiently and are the important species during ATP hydrolysis.
Figures




References
-
- Holland I. B., Blight M. A. ABC-ATPases, adaptable energy generators fuelling transmembrane movement of a variety of molecules in organisms from bacteria to humans. J. Mol. Biol. 1999;293:381–399. - PubMed
-
- Linton K., Higgins C. F. The Escherichia coli ATP-binding cassette (ABC) proteins. Mol. Microbiol. 1998;28:5–13. - PubMed
-
- Young J., Holland I. B. ABC transporters: bacterial exporters – revisited five years on. Biochim. Biophys. Acta. 1999;1461:177–200. - PubMed
-
- Holland I. B., Benabdelhak H., Young J., Pimenta A. L., Schmitt L., Blight M. Bacterial ABC transporters involved in protein translocation. In: Holland I. B., Cole S. P. C., Kuchler K., Higgins C. F., editors. ABC Proteins: From Bacteria to Man. London: Academic Press; 2003. pp. 209–241.
-
- Kerppola R. E., Shyamala V. K., Klebba P., Ames G. F.-L. The membrane-bound proteins of periplasmic permeases form a complex: identification of the histidine permease HisQMP complex. J. Biol. Chem. 1991;266:9857–9865. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

