IgE- and IgE+Ag-mediated mast cell migration in an autocrine/paracrine fashion
- PMID: 15637135
- PMCID: PMC1464406
- DOI: 10.1182/blood-2004-11-4205
IgE- and IgE+Ag-mediated mast cell migration in an autocrine/paracrine fashion
Abstract
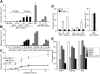
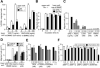
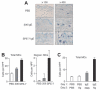
Mast cells are the major effector cells for immediate hypersensitivity and chronic allergic reactions. These cells accumulate in mucosal tissues of allergic reactions, where immunoglobulin E (IgE) is produced locally. Here we provide evidence that, in addition to antigen that can attract IgE-bound mast cells, the type of IgE molecules that efficiently activate mast cells can promote the migration of mast cells in the absence of antigen. IgE- and IgE+Ag-mediated migration involves an autocrine/paracrine secretion of soluble factors including adenosine, leukotriene B4, and several chemokines. Their secretion depends on 2 tyrosine kinases, Lyn and Syk, and they are agonists of G-protein-coupled receptors and signal through phosphatidylinositol 3-kinase gamma, leading to mast cell migration. In mouse experiments, naive mast cells are attracted to IgE, and IgE-sensitized mast cells are attracted to antigen. Therefore, IgE and antigen are implicated in mast cell accumulation at allergic tissue sites with local high IgE levels.
Figures


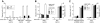
References
-
- Metzger H. The receptor with high affinity for IgE. Immunol Rev. 1992;125:37–48. - PubMed
-
- Kinet JP. The high-affinity IgE receptor (Fc∈;RI): from physiology to pathology. Annu Rev Immunol. 1999;17:931–972. - PubMed
-
- Turner H, Kinet JP. Signalling through the high-affinity IgE receptor Fc∈;RI. Nature. 1999;402:B24–30. - PubMed
-
- Rivera J. Molecular adapters in Fc∈;RI signaling and the allergic response. Curr Opin Immunol. 2002;14:688–693. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

