Distinct roles of insulin and liver X receptor in the induction and cleavage of sterol regulatory element-binding protein-1c
- PMID: 15637161
- PMCID: PMC545517
- DOI: 10.1073/pnas.0405067102
Distinct roles of insulin and liver X receptor in the induction and cleavage of sterol regulatory element-binding protein-1c
Abstract
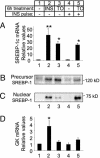
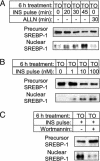
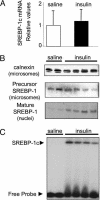
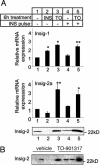
Sterol regulatory element-binding proteins (SREBPs) are transcription factors central to the regulation of lipid metabolism. The SREBPs are synthesized as precursor proteins that require proteolytic processing to become transcriptionally active. Whereas the regulation of SREBP-1a and -2 cleavage by cellular sterol content is well defined, much less is known about the regulation of SREBP-1c, the predominant SREBP isoform in the liver. Both insulin and liver X receptor alpha (LXRalpha) induce SREBP-1c transcription; however, the respective roles of these factors and the mechanism responsible for proteolytic cleavage of this SREBP isoform are not known. In this study, we compare the effects of insulin and LXR agonist TO-901317 on SREBP-1c expression and transcriptional activity in isolated rat hepatocytes. We report that full induction of the mature and transcriptionally active form of SREBP-1c protein requires insulin. Although activation of LXR leads to the induction of SREBP-1c gene expression and precursor protein, it has a very poor effect in inducing the mature nuclear form of the transcription factor. This may be due to the induction of insulin-induced gene-2a mRNA and protein by LXR activation. The LXR-induced SREBP-1c precursor, however, is rapidly cleaved on acute exposure to insulin via a phosphatidylinositol 3-kinase-dependent mechanism. Finally, we show through experiments in suckling mice that this acute action of insulin to stimulate the proteolytic processing of SREBP-1c is functional in vivo.
Figures

References
-
- Shimano, H., Yahagi, N., Amemiya-Kudo, M., Hasty, A. H., Osuga, J., Tamura, Y., Shionoiri, F., Iizuka, Y., Ohashi, K., Harada, K., et al. (1999) J. Biol. Chem. 274, 35832-35839. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

