Hepatically derived selenoprotein P is a key factor for kidney but not for brain selenium supply
- PMID: 15638810
- PMCID: PMC1134785
- DOI: 10.1042/BJ20041973
Hepatically derived selenoprotein P is a key factor for kidney but not for brain selenium supply
Abstract
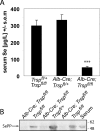
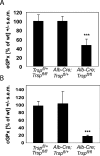
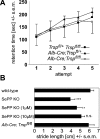
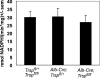
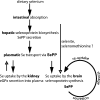
Liver-specific inactivation of Trsp, the gene for selenocysteine tRNA, removes SePP (selenoprotein P) from plasma, causing serum selenium levels to fall from 298 microg/l to 50 microg/l and kidney selenium to decrease to 36% of wild-type levels. Likewise, glutathione peroxidase activities decreased in plasma and kidney to 43% and 18% respectively of wild-type levels. This agrees nicely with data from SePP knockout mice, supporting a selenium transport role for hepatically expressed SePP. However, brain selenium levels remain unaffected and neurological defects do not occur in the liver-specific Trsp knockout mice, while SePP knockout mice suffer from neurological defects. This indicates that a transport function in plasma is exerted by hepatically derived SePP, while in brain SePP fulfils a second, hitherto unexpected, essential role.
Figures

Comment in
-
More roles for selenoprotein P: local selenium storage and recycling protein in the brain.Biochem J. 2005 Mar 1;386(Pt 2):e5-7. doi: 10.1042/BJ20050149. Biochem J. 2005. PMID: 15720294 Free PMC article. Review.
References
-
- Rayman M. P. The importance of selenium to human health. Lancet. 2000;356:233–241. - PubMed
-
- Kryukov G. V., Castellano S., Novoselov S. V., Lobanov A. V., Zehtab O., Guigo R., Gladyshev V. N. Characterization of mammalian selenoproteomes. Science. 2003;300:1439–1443. - PubMed
-
- Brigelius-Flohé R. Tissue-specific functions of individual glutathione peroxidases. Free Radical Biol. Med. 1999;27:951–965. - PubMed
-
- Rundlof A. K., Arner E. S. Regulation of the mammalian selenoprotein thioredoxin reductase 1 in relation to cellular phenotype, growth, and signaling events. Antioxid. Redox Signalling. 2004;6:41–52. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

