Autotrophic ammonia-oxidizing bacteria contribute minimally to nitrification in a nitrogen-impacted forested ecosystem
- PMID: 15640188
- PMCID: PMC544198
- DOI: 10.1128/AEM.71.1.197-206.2005
Autotrophic ammonia-oxidizing bacteria contribute minimally to nitrification in a nitrogen-impacted forested ecosystem
Abstract
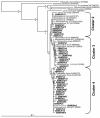
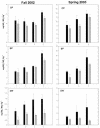
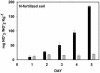
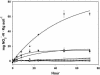
Deposition rates of atmospheric nitrogenous pollutants to forests in the San Bernardino Mountains range east of Los Angeles, California, are the highest reported in North America. Acidic soils from the west end of the range are N-saturated and have elevated rates of N-mineralization, nitrification, and nitrate leaching. We assessed the impact of this heavy nitrogen load on autotrophic ammonia-oxidizing communities by investigating their composition, abundance, and activity. Analysis of 177 cloned beta-Proteobacteria ammonia oxidizer 16S rRNA genes from highly to moderately N-impacted soils revealed similar levels of species composition; all of the soils supported the previously characterized Nitrosospira clusters 2, 3, and 4. Ammonia oxidizer abundance measured by quantitative PCR was also similar among the soils. However, rates of potential nitrification activity were greater for N-saturated soils than for soils collected from a less impacted site, but autotrophic (i.e., acetylene-sensitive) activity was low in all soils examined. N-saturated soils incubated for 30 days with ammonium accumulated additional soluble ammonium, whereas less-N-impacted soils had a net loss of ammonium. Lastly, nitrite production by cultivated Nitrosospira multiformis, an autotrophic ammonia-oxidizing bacterium adapted to relatively high ammonium concentrations, was significantly inhibited in pH-controlled slurries of sterilized soils amended with ammonium despite the maintenance of optimal ammonia-oxidizing conditions. Together, these results showed that factors other than autotrophic ammonia oxidizers contributed to high nitrification rates in these N-impacted forest soils and, unlike many other environments, differences in nitrogen content and soil pH did not favor particular autotrophic ammonia oxidizer groups.
Figures
References
-
- Aber, J. D., P. Nadelhoffer, P. Steudler, and J. M. Melillo. 1989. Nitrogen saturation in northern forest ecosystems. BioScience 39:378-386.
-
- Arkley, R. J. 1981. Soil-moisture use by mixed conifer forest in a summer-dry climate. Soil Sci. Soc. Am. J. 45:423-427.
-
- Bothe, H., G. Jost, M. Schloter, B. B. Ward, and K.-P. Witzel. 2000. Molecular analysis of ammonia oxidation and denitrification in natural environments. FEMS Microbiol. Rev. 24:673-690. - PubMed
-
- Bouwman, A. F., D. P. Van Vuuren, R. G. Derwent, and M. Posch. 2002. A global analysis of acidification and eutrophication of terrestrial ecosystems. Water Air Soil Pollut. 141:349-382.
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

