Green fluorescent protein is a quantitative reporter of gene expression in individual eukaryotic cells
- PMID: 15640280
- PMCID: PMC1242169
- DOI: 10.1096/fj.04-3180fje
Green fluorescent protein is a quantitative reporter of gene expression in individual eukaryotic cells
Abstract
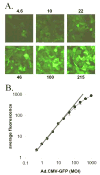
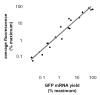
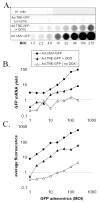
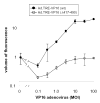
Green fluorescent protein (GFP) has gained widespread use as a tool to visualize spatial and temporal patterns of gene expression in vivo. However, it is not generally accepted that GFP can also be used as a quantitative reporter of gene expression. We report that GFP is a reliable reporter of gene expression in individual eukaryotic cells when fluorescence is measured by flow cytometry. Two pieces of evidence support this conclusion: GFP fluorescence increases in direct proportion to the GFP gene copy number delivered to cells by a replication-defective adenovirus vector, Ad.CMV-GFP, and the intensity of GFP fluorescence is directly proportional to GFP mRNA abundance in cells. This conclusion is further supported by the fact that the induction of GFP gene expression from two inducible promoters (i.e., the TRE and ICP0 promoters) is readily detected by flow cytometric measurement of GFP fluorescent intensity. Collectively, the results presented herein indicate that GFP fluorescence is a reliable and quantitative reporter of underlying differences in gene expression.
Figures


References
-
- Aints A, Dilber MS, Smith CI. Intercellular spread of GFP-VP22. J Gene Med. 1999;1:275–9. - PubMed
-
- Albano CR, Randers-Eichhorn L, Bentley WE, Rao G. Green fluorescent protein as a real time quantitative reporter of heterologous protein production. Biotechnol Prog. 1998;14:351–4. - PubMed
-
- Baulcombe DC, Chapman S, Santa Cruz S. Jellyfish green fluorescent protein as a reporter for virus infections. Plant J. 1995;7:1045–53. - PubMed
-
- Boes M, Cerny J, Massol R, Op den Brouw M, Kirchhausen T, Chen J, Ploegh HL. T-cell engagement of dendritic cells rapidly rearranges MHC class II transport. Nature. 2002;418:983–8. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

