The effect of drotrecogin alfa (activated) on long-term survival after severe sepsis
- PMID: 15640631
- PMCID: PMC4714718
- DOI: 10.1097/01.ccm.0000145228.62451.f6
The effect of drotrecogin alfa (activated) on long-term survival after severe sepsis
Abstract
Objective: To determine long-term survival for subjects with severe sepsis enrolled in the previous multiple-center trial (PROWESS) of drotrecogin alfa (activated) (DrotAA) vs. placebo.
Design: Retrospective, cross-sectional, blinded follow-up of subjects enrolled in a previous randomized, controlled trial.
Setting: One hundred sixty-four tertiary care institutions in 11 countries.
Participants: The 1,690 subjects with severe sepsis enrolled and treated with study drug in PROWESS, of whom 1,220 were alive at 28 days (the end of the original PROWESS follow-up).
Interventions: DrotAA (n = 850), 24 mug/kg/hr for 96 hrs, or placebo (n = 840).
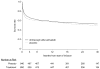
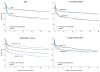
Measurements and main results: Long-term survival data were collected. We had follow-up information on 100% of subjects at 28 days, 98% at hospital discharge, 94% at 3 months, and 93% at 1 yr. The longest follow-up was 3.6 yrs. Hospital survival was higher with DrotAA vs. placebo (70.3% vs. 65.1%, p = .03). There was no statistically significant difference in duration of survival time or in landmark survival rates in subjects who received DrotAA compared with those who received placebo (median duration of survival = 1113 days vs. 846 days for DrotAA vs. placebo, p = .10; landmark survival rates for DrotAA vs. placebo, 66.1% vs. 62.4% at 3 months [p = .11], 62.2% vs. 60.3% at 6 months [p = .44], 58.9% vs. 57.2% at 1 yr [p = .49], and 52.6% vs. 49.3% at 2(1/2) yrs [p = .21]). There was a significant interaction (p = .0008) between treatment assignment and baseline Acute Physiology and Chronic Health Evaluation (APACHE) II scores, suggesting qualitative differences in treatment effect with severity of illness. Subjects with APACHE II >/=25 had better survival time with DrotAA (median duration of survival: 450 vs. 71 days, p =.0005). Survival rates were also higher at landmark time points (DrotAA vs. placebo, 58.9% vs. 48.4% at 3 months [p = .003], 55.2% vs. 45.3% at 6 months [p = .005], 52.1% vs. 41.3% at 1 yr [p = .002], and 45.6% vs. 33.8% at 2(1/2) yrs [p = .001]). In the APACHE II <25 group there was no significant difference in survival time or survival rates at landmark time points except at 1 yr (DrotAA vs. placebo, 65.5% vs. 72.0% at 1 yr, p = .04).
Conclusions: The acute survival benefit observed in subjects with severe sepsis who received DrotAA persists to hospital discharge. The survival benefit loses statistical significance thereafter. Post hoc analysis suggests the effect of DrotAA varies by APACHE II score with improved long-term survival in subjects with APACHE II scores >/=25 but no benefit in those with lower scores.
Figures
Comment in
-
The efficacy of drotrecogin alfa depends on severity of illness.Crit Care Med. 2004 Nov;32(11):2347. doi: 10.1097/01.ccm.0000146002.38054.70. Crit Care Med. 2004. PMID: 15640655 No abstract available.
-
Activated protein C: beyond 28 days.Crit Care Med. 2004 Nov;32(11):2348-9. doi: 10.1097/01.ccm.0000146137.32072.f7. Crit Care Med. 2004. PMID: 15640656 No abstract available.
References
-
- American College of Chest Physicians/Society of Critical Care Medicine Consensus Conference: definitions for sepsis and organ failure and guidelines for the use of innovative therapies in sepsis. Crit Care Med. 1992;20:864–874. - PubMed
-
- Angus DC, Linde-Zwirble WT, Lidicker J, et al. Epidemiology of severe sepsis in the United States: analysis of incidence, outcome, and associated costs of care. Crit Care Med. 2001;29:1303–1310. - PubMed
-
- Padkin A, Goldfrad C, Brady AR, et al. Epidemiology of severe sepsis occurring in the first 24 hours in the ICU in England, Wales, and Northern Ireland. Crit Care Med. 2003;9:2332–2338. - PubMed
-
- Brun-Buisson C, Doyon F, Carlet J, et al. Incidence, risk factors, and outcome of severe sepsis and septic shock in adults. A multicenter prospective study in intensive care units. French ICU Group for Severe Sepsis. JAMA. 1995;274:968–974. - PubMed
-
- Davies A, Green C, Hutton J, et al. (2001) Severe sepsis: a European estimate of the burden of disease in ICU. Intensive Care Med. 2001;27:S284. Abstract 581.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

