New combination test for hepatitis C virus genotype and viral load determination using Amplicor GT HCV MONITOR test v2.0
- PMID: 15641128
- PMCID: PMC4250793
- DOI: 10.3748/wjg.v11.i4.469
New combination test for hepatitis C virus genotype and viral load determination using Amplicor GT HCV MONITOR test v2.0
Abstract
Aim: To develop a new sensitive and inexpensive hepatitis C virus (HCV) combination test (HCV Guideline test) that enables the determination of HCV genotypes 1, 2 and 3, and simultaneous determination of HCV viral load using commercial Amplicor GT HCV MONITOR test v2.0 (microwell version).
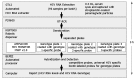
Methods: The HCV Guideline test used the PCR product generated in commercial Amplicor GT HCV Monitor test v2.0 for viral load measurement using microwell plate version of Amplicor HCV Monitor and also captured on separate plates containing capture probes and competitive oligonucleotide probes specific for HCV genotypes 1, 2 and 3, The HCV genotype was subsequently determined using the biotin-labeled PCR product and five biotin-labeled HCV-specific probes.
Results: The sensitivity of the HCV Guideline test was 0.5 KIU/mL. Specificity of the HCV Guideline test was confirmed by direct sequencing of HCV core region and molecular evolutionary analyses based on a panel of 31 samples. The comparison of the HCV Guideline test and an in-house HCV core genotyping assay using 252 samples from chronic hepatitis C patients indicated concordant results for 97.2% of samples (59.5% genotype 1, 33.7% genotype 2, 6.0% genotype 3, and 0.8% mixed genotypes). Similarly, the HCV Guideline test showed concordance with a serological test, and the serological test failed to assign any serotype in 12.7% of the samples, indicating a better sensitivity of the HCV Guideline test.
Conclusion: Clinically, both viral load and genotypes (1, 2 and 3) have been found to be major predictors of antiviral therapy outcome regarding chronic hepatitis C based on guidelines and they are, in normal circumstances, performed as separate stand-alone assays. The HCV Guideline test is a useful method for screening large cohorts in a routine clinical setting for determining the treatment regimen and for predicting the outcome of antiviral therapy of chronic hepatitis C.
Figures


Similar articles
-
Evaluation of the clinical usefulness of COBAS AMPLICOR HCV MONITOR assay (ver2.0): Comparison with AMPLICOR HCV MONITOR assay (ver1.0) and HCV core protein level.J Med Virol. 2002 Nov;68(3):343-51. doi: 10.1002/jmv.10209. J Med Virol. 2002. PMID: 12226820
-
Comparative evaluation of the total hepatitis C virus core antigen, branched-DNA, and amplicor monitor assays in determining viremia for patients with chronic hepatitis C during interferon plus ribavirin combination therapy.J Clin Microbiol. 2003 Jul;41(7):3212-20. doi: 10.1128/JCM.41.7.3212-3220.2003. J Clin Microbiol. 2003. PMID: 12843066 Free PMC article.
-
Comparison of qualitative (COBAS AMPLICOR HCV 2.0 versus VERSANT HCV RNA) and quantitative (COBAS AMPLICOR HCV monitor 2.0 versus VERSANT HCV RNA 3.0) assays for hepatitis C virus (HCV) RNA detection and quantification: impact on diagnosis and treatment of HCV infections.J Clin Microbiol. 2005 Jun;43(6):2590-7. doi: 10.1128/JCM.43.6.2590-2597.2005. J Clin Microbiol. 2005. PMID: 15956369 Free PMC article.
-
[Viral safety of biologicals: evaluation of hepatitis C virus (HCV) nucleic acid amplification test (NAT) assay and development of concentration method of HCV for sensitive detection by NAT].Yakugaku Zasshi. 2010 Feb;130(2):163-9. doi: 10.1248/yakushi.130.163. Yakugaku Zasshi. 2010. PMID: 20118638 Review. Japanese.
-
Molecular diagnostic and predictive tests in the evolution of chronic hepatitis C anti-viral therapies.BMC Infect Dis. 2012;12 Suppl 2(Suppl 2):S8. doi: 10.1186/1471-2334-12-S2-S8. Epub 2012 Nov 12. BMC Infect Dis. 2012. PMID: 23173776 Free PMC article. Review.
References
-
- Alter HJ, Purcell RH, Shih JW, Melpolder JC, Houghton M, Choo QL, Kuo G. Detection of antibody to hepatitis C virus in prospectively followed transfusion recipients with acute and chronic non-A, non-B hepatitis. N Engl J Med. 1989;321:1494–1500. - PubMed
-
- Wasley A, Alter MJ. Epidemiology of hepatitis C: geographic differences and temporal trends. Semin Liver Dis. 2000;20:1–16. - PubMed
-
- Tanaka Y, Hanada K, Mizokami M, Yeo AE, Shih JW, Gojobori T, Alter HJ. A comparison of the molecular clock of hepatitis C virus in the United States and Japan predicts that hepatocellular carcinoma incidence in the United States will increase over the next two decades. Proc Natl Acad Sci USA. 2002;99:15584–15589. - PMC - PubMed
-
- Robertson B, Myers G, Howard C, Brettin T, Bukh J, Gaschen B, Gojobori T, Maertens G, Mizokami M, Nainan O, et al. Classification, nomenclature, and database development for hepatitis C virus (HCV) and related viruses: proposals for standardization. International Committee on Virus Taxonomy. Arch Virol. 1998;143:2493–2503. - PubMed
-
- Orito E, Mizokami M, Nakano T, Terashima H, Nojiri O, Sakakibara K, Mizuno M, Ogino M, Nakamura M, Matsumoto Y. Serum hepatitis C virus RNA level as a predictor of subsequent response to interferon-alpha therapy in Japanese patients with chronic hepatitis C. J Med Virol. 1994;44:410–414. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

