Relaxin's induction of metalloproteinases is associated with the loss of collagen and glycosaminoglycans in synovial joint fibrocartilaginous explants
- PMID: 15642129
- PMCID: PMC1064880
- DOI: 10.1186/ar1451
Relaxin's induction of metalloproteinases is associated with the loss of collagen and glycosaminoglycans in synovial joint fibrocartilaginous explants
Abstract
Diseases of specific fibrocartilaginous joints are especially common in women of reproductive age, suggesting that female hormones contribute to their etiopathogenesis. Previously, we showed that relaxin dose-dependently induces matrix metalloproteinase (MMP) expression in isolated joint fibrocartilaginous cells. Here we determined the effects of relaxin with or without beta-estradiol on the modulation of MMPs in joint fibrocartilaginous explants, and assessed the contribution of these proteinases to the loss of collagen and glycosaminoglycan (GAG) in this tissue. Fibrocartilaginous discs from temporomandibular joints of female rabbits were cultured in medium alone or in medium containing relaxin (0.1 ng/ml) or beta-estradiol (20 ng/ml) or relaxin plus beta-estradiol. Additional experiments were done in the presence of the MMP inhibitor GM6001 or its control analog. After 48 hours of culture, the medium was assayed for MMPs and the discs were analyzed for collagen and GAG concentrations. Relaxin and beta-estradiol plus relaxin induced the MMPs collagenase-1 and stromelysin-1 in fibrocartilaginous explants--a finding similar to that which we observed in pubic symphysis fibrocartilage, but not in articular cartilage explants. The induction of these proteinases by relaxin or beta-estradiol plus relaxin was accompanied by a loss of GAGs and collagen in joint fibrocartilage. None of the hormone treatments altered the synthesis of GAGs, suggesting that the loss of this matrix molecule probably resulted from increased matrix degradation. Indeed, fibrocartilaginous explants cultured in the presence of GM6001 showed an inhibition of relaxin-induced and beta-estradiol plus relaxin-induced collagenase and stromelysin activities to control baseline levels that were accompanied by the maintenance of collagen or GAG content at control levels. These findings show for the first time that relaxin has degradative effects on non-reproductive synovial joint fibrocartilaginous tissue and provide evidence for a link between relaxin, MMPs, and matrix degradation.
Figures


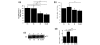
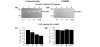

Similar articles
-
Matrix metalloproteinase induction by relaxin causes cartilage matrix degradation in target synovial joints.Ann N Y Acad Sci. 2009 Apr;1160:322-8. doi: 10.1111/j.1749-6632.2009.03830.x. Ann N Y Acad Sci. 2009. PMID: 19416213 Free PMC article.
-
Relaxin and beta-estradiol modulate targeted matrix degradation in specific synovial joint fibrocartilages: progesterone prevents matrix loss.Arthritis Res Ther. 2006;8(4):R98. doi: 10.1186/ar1978. Arthritis Res Ther. 2006. PMID: 16784544 Free PMC article.
-
Targeted induction of collagenase and stromelysin by relaxin in unprimed and beta-estradiol-primed diarthrodial joint fibrocartilaginous cells but not in synoviocytes.Lab Invest. 1998 Aug;78(8):925-38. Lab Invest. 1998. PMID: 9714180
-
The role of collagenolytic matrix metalloproteinases in the loss of articular cartilage in osteoarthritis.Front Biosci. 2006 Sep 1;11:3081-95. doi: 10.2741/2034. Front Biosci. 2006. PMID: 16720377 Review.
-
Review of the Multiscale Effects of Female Sex Hormones on Matrix Metalloproteinase-Mediated Collagen Degradation.Crit Rev Biomed Eng. 2015;43(5-6):401-28. doi: 10.1615/CritRevBiomedEng.2016016590. Crit Rev Biomed Eng. 2015. PMID: 27480583 Review.
Cited by
-
Relaxin as a treatment for musculoskeletal fibrosis: What we know and future directions.Biochem Pharmacol. 2024 Jul;225:116273. doi: 10.1016/j.bcp.2024.116273. Epub 2024 May 8. Biochem Pharmacol. 2024. PMID: 38729446 Free PMC article. Review.
-
17β-estradiol Induces MMP-9 and MMP-13 in TMJ Fibrochondrocytes via Estrogen Receptor α.J Dent Res. 2018 Aug;97(9):1023-1030. doi: 10.1177/0022034518767108. Epub 2018 Apr 5. J Dent Res. 2018. PMID: 29621430 Free PMC article.
-
Knee Laxities Changes with Sex-steroids throughout the Menstrual Cycle Phases in Athlete and Non-athlete Females.Rev Bras Ortop (Sao Paulo). 2024 Mar 21;59(1):e29-e37. doi: 10.1055/s-0043-1771007. eCollection 2024 Feb. Rev Bras Ortop (Sao Paulo). 2024. PMID: 38524710 Free PMC article.
-
Matrix metalloproteinase induction by relaxin causes cartilage matrix degradation in target synovial joints.Ann N Y Acad Sci. 2009 Apr;1160:322-8. doi: 10.1111/j.1749-6632.2009.03830.x. Ann N Y Acad Sci. 2009. PMID: 19416213 Free PMC article.
-
Relationship of relaxin hormone and thumb carpometacarpal joint arthritis.Clin Orthop Relat Res. 2014 Apr;472(4):1130-7. doi: 10.1007/s11999-013-2960-4. Clin Orthop Relat Res. 2014. PMID: 23559157 Free PMC article.
References
-
- Giori NJ, Beaupre GS, Carter DR. Cellular shape and pressure may mediate mechanical control of tissue composition in tendons. J Orthop Res. 1993;11:581–591. - PubMed
-
- Mills DK, Daniel JC. Development of functional specialization within the maturing rabbit flexor digitorum tendon. Connect Tissue Res. 1993;30:37–57. - PubMed
-
- Steinetz BG, O'Byrne EM, Butler MC, Hickman LB. Hormonal regulation of connective tissue of the symphysis pubis. In: Bigazzi M, Greenwood FC, Gaspari F, editor. In Biology of Relaxin and Its Role in the Human. Amsterdam: Excerpta Medica; 1983. pp. 71–92.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

