Mast cells in inflammatory arthritis
- PMID: 15642148
- PMCID: PMC1064877
- DOI: 10.1186/ar1446
Mast cells in inflammatory arthritis
Abstract
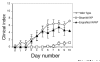
Mast cells are present in limited numbers in normal human synovium, but in rheumatoid arthritis and other inflammatory joint diseases this population can expand to constitute 5% or more of all synovial cells. Recent investigations in a murine model have demonstrated that mast cells can have a critical role in the generation of inflammation within the joint. This finding highlights the results of more than 20 years of research indicating that mast cells are frequent participants in non-allergic immune responses as well as in allergy. Equipped with a diversity of surface receptors and effector capabilities, mast cells are sentinels of the immune system, detecting and delivering a first response to invading bacteria and other insults. Accumulating within inflamed tissues, mast cells produce cytokines and other mediators that may contribute vitally to ongoing inflammation. Here we review some of the non-allergic functions of mast cells and focus on the potential role of these cells in murine and human inflammatory arthritis.
Figures


References
-
- Lee DM, Friend DS, Gurish MF, Benoist C, Mathis D, Brenner MB. Mast cells: a cellular link between autoantibodies and inflammatory arthritis. Science. 2002;297:1689–1692. - PubMed
-
- Corr M, Crain B. The role of FcγR signaling in the K/B x N serum transfer model of arthritis. J Immunol. 2002;169:6604–6669. - PubMed
-
- Metcalfe DD, Baram D, Mekori YA. Mast cells. Physiol Rev. 1997;77:1033–1079. - PubMed
-
- Kirshenbaum AS, Kessler SW, Goff JP, Metcalfe DD. Demonstration of the origin of human mast cells from CD34+ bone marrow progenitor cells. J Immunol. 1991;146:1410–1415. - PubMed
-
- Rodewald HR, Dessing M, Dvorak AM, Galli SJ. Identification of a committed precursor for the mast cell lineage. Science. 1996;271:818–822. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

