Breast fibroblasts modulate epithelial cell proliferation in three-dimensional in vitro co-culture
- PMID: 15642169
- PMCID: PMC1064098
- DOI: 10.1186/bcr949
Breast fibroblasts modulate epithelial cell proliferation in three-dimensional in vitro co-culture
Abstract
Background: Stromal fibroblasts associated with in situ and invasive breast carcinoma differ phenotypically from fibroblasts associated with normal breast epithelium, and these alterations in carcinoma-associated fibroblasts (CAF) may promote breast carcinogenesis and cancer progression. A better understanding of the changes that occur in fibroblasts during carcinogenesis and their influence on epithelial cell growth and behavior could lead to novel strategies for the prevention and treatment of breast cancer. To this end, the effect of CAF and normal breast-associated fibroblasts (NAF) on the growth of epithelial cells representative of pre-neoplastic breast disease was assessed.
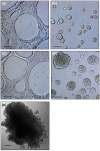
Methods: NAF and CAF were grown with the nontumorigenic MCF10A epithelial cells and their more transformed, tumorigenic derivative, MCF10AT cells, in direct three-dimensional co-cultures on basement membrane material. The proliferation and apoptosis of MCF10A cells and MCF10AT cells were assessed by 5-bromo-2'-deoxyuridine labeling and TUNEL assay, respectively. Additionally, NAF and CAF were compared for expression of insulin-like growth factor II as a potential mediator of their effects on epithelial cell growth, by ELISA and by quantitative, real-time PCR.
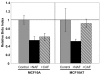
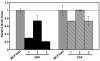
Results: In relatively low numbers, both NAF and CAF suppressed proliferation of MCF10A cells. However, only NAF and not CAF significantly inhibited proliferation of the more transformed MCF10AT cells. The degree of growth inhibition varied among NAF or CAF from different individuals. In greater numbers, NAF and CAF have less inhibitory effect on epithelial cell growth. The rate of epithelial cell apoptosis was not affected by NAF or CAF. Mean insulin-like growth factor II levels were not significantly different in NAF versus CAF and did not correlate with the fibroblast effect on epithelial cell proliferation.
Conclusion: Both NAF and CAF have the ability to inhibit the growth of pre-cancerous breast epithelial cells. NAF have greater inhibitory capacity than CAF, suggesting that the ability of fibroblasts to inhibit epithelial cell proliferation is lost during breast carcinogenesis. Furthermore, as the degree of transformation of the epithelial cells increased they became resistant to the growth-inhibitory effects of CAF. Insulin-like growth factor II could not be implicated as a contributor to this differential effect of NAF and CAF on epithelial cell growth.
Figures





References
-
- Kunz-Schughart LA, Knuechel R. Tumor-associated fibroblasts (part II): functional impact on tumor tissue. Histol Histopathol. 2002;17:623–637. - PubMed
-
- Kunz-Schughart LA, Knuechel R. Tumor-associated fibroblasts (part I): active stromal participants in tumor development and progression? Histol Histopathol. 2002;17:599–621. - PubMed
-
- Heffelfinger SC, Miller MA, Yassin R, Gear R. Angiogenic growth factors in preinvasive breast disease. Clin Cancer Res. 1999;5:2867–2876. - PubMed
-
- Costantini V, Sidoni A, Deveglia R, Cazzato OA, Bellezza G, Ferri I, Bucciarelli E, Nenci GG. Combined overexpression of urokinase, urokinase receptor, and plasminogen activator inhibitor-1 is associated with breast cancer progression: an immunohistochemical comparison of normal, benign, and malignant breast tissues. Cancer. 1996;77:1079–1088. doi: 10.1002/(SICI)1097-0142(19960315)77:6<1079::AID-CNCR12>3.0.CO;2-Z. - DOI - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

