Prenylation inhibitors stimulate both estrogen receptor alpha transcriptional activity through AF-1 and AF-2 and estrogen receptor beta transcriptional activity
- PMID: 15642170
- PMCID: PMC1064103
- DOI: 10.1186/bcr956
Prenylation inhibitors stimulate both estrogen receptor alpha transcriptional activity through AF-1 and AF-2 and estrogen receptor beta transcriptional activity
Abstract
Introduction: We showed in a previous study that prenylated proteins play a role in estradiol stimulation of proliferation. However, these proteins antagonize the ability of estrogen receptor (ER) alpha to stimulate estrogen response element (ERE)-dependent transcriptional activity, potentially through the formation of a co-regulator complex. The present study investigates, in further detail, how prenylated proteins modulate the transcriptional activities mediated by ERalpha and by ERbeta.
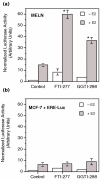
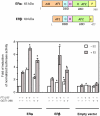
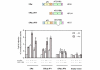
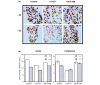
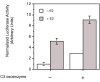
Methods: The ERE-beta-globin-Luc-SV-Neo plasmid was either stably transfected into MCF-7 cells or HeLa cells (MELN cells and HELN cells, respectively) or transiently transfected into MCF-7 cells using polyethylenimine. Cells deprived of estradiol were analyzed for ERE-dependent luciferase activity 16 hours after estradiol stimulation and treatment with FTI-277 (a farnesyltransferase inhibitor) or with GGTI-298 (a geranylgeranyltransferase I inhibitor). In HELN cells, the effect of prenyltransferase inhibitors on luciferase activity was compared after transient transfection of plasmids coding either the full-length ERalpha, the full-length ERbeta, the AF-1-deleted ERalpha or the AF-2-deleted ERalpha. The presence of ERalpha was then detected by immunocytochemistry in either the nuclei or the cytoplasms of MCF-7 cells. Finally, Clostridium botulinum C3 exoenzyme treatment was used to determine the involvement of Rho proteins in ERE-dependent luciferase activity.
Results: FTI-277 and GGTI-298 only stimulate ERE-dependent luciferase activity in stably transfected MCF-7 cells. They stimulate both ERalpha-mediated and ERbeta-mediated ERE-dependent luciferase activity in HELN cells, in the presence of and in the absence of estradiol. The roles of both AF-1 and AF-2 are significant in this effect. Nuclear ERalpha is decreased in the presence of prenyltransferase inhibitors in MCF-7 cells, again in the presence of and in the absence of estradiol. By contrast, cytoplasmic ERalpha is mainly decreased after treatment with FTI-277, in the presence of and in the absence of estradiol. The involvement of Rho proteins in ERE-dependent luciferase activity in MELN cells is clearly established.
Conclusions: Together, these results demonstrate that prenylated proteins (at least RhoA, RhoB and/or RhoC) antagonize the ability of ERalpha and ERbeta to stimulate ERE-dependent transcriptional activity, potentially acting through both AF-1 and AF-2 transcriptional activities.
Figures
Similar articles
-
Transcriptional regulation of vascular endothelial growth factor by estradiol and tamoxifen in breast cancer cells: a complex interplay between estrogen receptors alpha and beta.Cancer Res. 2002 Sep 1;62(17):4977-84. Cancer Res. 2002. PMID: 12208749
-
Contrasting effects of prenyltransferase inhibitors on estrogen-dependent cell cycle progression and estrogen receptor-mediated transcriptional activity in MCF-7 cells.Endocrinology. 2003 Mar;144(3):989-98. doi: 10.1210/en.2002-220726. Endocrinology. 2003. PMID: 12586776
-
Evaluation of ligand selectivity using reporter cell lines stably expressing estrogen receptor alpha or beta.Biochem Pharmacol. 2006 May 14;71(10):1459-69. doi: 10.1016/j.bcp.2006.02.002. Epub 2006 Mar 22. Biochem Pharmacol. 2006. PMID: 16554039
-
From ligand structure to biological activity: modified estratrienes and their estrogenic and antiestrogenic effects in MCF-7 cells.Steroids. 2004 Jun;69(6):401-18. doi: 10.1016/j.steroids.2004.03.014. Steroids. 2004. PMID: 15219790 Review.
-
Histone deacetylase inhibition and estrogen signalling in human breast cancer cells.Biochem Pharmacol. 2004 Sep 15;68(6):1239-46. doi: 10.1016/j.bcp.2004.04.031. Biochem Pharmacol. 2004. PMID: 15313422 Review.
Cited by
-
RhoA and RhoC differentially modulate estrogen receptor α recruitment, transcriptional activities, and expression in breast cancer cells (MCF-7).J Cancer Res Clin Oncol. 2013 Dec;139(12):2079-88. doi: 10.1007/s00432-013-1533-y. Epub 2013 Oct 6. J Cancer Res Clin Oncol. 2013. PMID: 24096540 Free PMC article.
References
-
- Webb P, Nguyen P, Valentine C, Lopez GN, Kwok GR, McInerney E, Katzenellenbogen BS, Enmark E, Gustafsson JA, Nilsson S, Kushner PJ. The estrogen receptor enhances AP-1 activity by two distinct mechanisms with different requirements for receptor transactivation functions. Mol Endocrinol. 1999;13:1672–1685. doi: 10.1210/me.13.10.1672. - DOI - PubMed
-
- Ogawa S, Inoue S, Watanabe T, Hiroi H, Orimo A, Hosoi T, Ouchi Y, Muramatsu M. The complete primary structure of human estrogen receptor beta (hER beta) and its heterodimerization with ER alpha in vivo and in vitro. Biochem Biophys Res Commun. 1998;243:122–126. doi: 10.1006/bbrc.1997.7893. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

