Cotranslational endoplasmic reticulum assembly of FcepsilonRI controls the formation of functional IgE-binding receptors
- PMID: 15642744
- PMCID: PMC2212795
- DOI: 10.1084/jem.20041384
Cotranslational endoplasmic reticulum assembly of FcepsilonRI controls the formation of functional IgE-binding receptors
Abstract
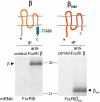
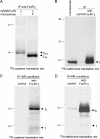
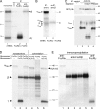
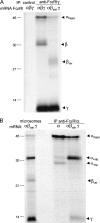
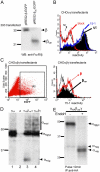
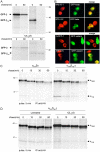
The human high affinity receptor for IgE (FcepsilonRI) is a cell surface structure critical for the pathology of allergic reactions. Human FcepsilonRI is expressed as a tetramer (alphabetagamma(2)) on basophils or mast cells and as trimeric (alphagamma(2)) complex on antigen-presenting cells. Expression of the human alpha subunit can be down-regulated by a splice variant of FcepsilonRIbeta (beta(var)). We demonstrate that FcepsilonRIalpha is the core subunit with which the other subunits assemble strictly cotranslationally. In addition to alphabetagamma(2) and alphagamma(2), we demonstrate the presence of alphabeta and alphabeta(var)gamma(2) complexes that are stable in the detergent Brij 96. The role of individual FcepsilonRI subunits for the formation of functional, immunoglobulin E-binding FcepsilonRI complexes during endoplasmic reticulum (ER) assembly can be defined as follows: beta and gamma support ER insertion, signal peptide cleavage and proper N-glycosylation of alpha, whereas beta(var) allows accumulation of alpha protein backbone. We show that assembly of FcepsilonRI in the ER is a key step for the regulation of surface expression of FcepsilonRI. The ER quality control system thus regulates the quantity of functional FcepsilonRI, which in turn controls onset and persistence of allergic reactions.
Figures


References
-
- Sutton, B.J., and H.J. Gould. 1993. The human IgE network. Nature. 366:421–428. - PubMed
-
- Cookson, W.O., and M.F. Moffatt. 1997. Asthma: an epidemic in the absence of infection? Science. 275:41–42. - PubMed
-
- Metzger, H. 1991. The high affinity receptor for IgE on mast cells. Clin. Exp. Allergy. 21:269–279. - PubMed
-
- Mudde, G.C., I.G. Reischl, N. Corvaia, A. Hren, and E.M. Poellabauer. 1996. Antigen presentation in allergic sensitization. Immunol. Cell Biol. 74:167–173. - PubMed
-
- Kinet, J.P. 1999. The high-affinity IgE receptor (Fc epsilon RI): from physiology to pathology. Annu. Rev. Immunol. 17:931–972. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

