Gp135/podocalyxin and NHERF-2 participate in the formation of a preapical domain during polarization of MDCK cells
- PMID: 15642748
- PMCID: PMC2171597
- DOI: 10.1083/jcb.200407072
Gp135/podocalyxin and NHERF-2 participate in the formation of a preapical domain during polarization of MDCK cells
Abstract
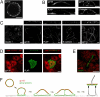
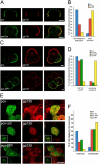
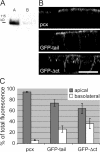
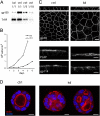
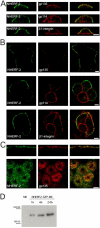
Epithelial polarization involves the segregation of apical and basolateral membrane domains, which are stabilized and maintained by tight junctions and membrane traffic. We report that unlike most apical and basolateral proteins in MDCK cells, which separate only after junctions have formed, the apical marker gp135 signifies an early level of polarized membrane organization established already in single cells. We identified gp135 as the dog orthologue of podocalyxin. With a series of domain mutants we show that the COOH-terminal PSD-95/Dlg/ZO-1 (PDZ)-binding motif is targeting podocalyxin to the free surface of single cells as well as to a subdomain of the terminally polarized apical membrane. This special localization of podocalyxin is shared by the cytoplasmic PDZ-protein Na+/H+ exchanger regulatory factor (NHERF)-2. Depleting podocalyxin by RNA interference caused defects in epithelial polarization. Together, our data suggest that podocalyxin and NHERF-2 function in epithelial polarization by contributing to an early apical scaffold based on PDZ domain-mediated interactions.
Figures
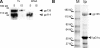


References
-
- Baas, A.F., J. Kuipers, N.N. van der Wel, E. Batlle, H.K. Koerten, P.J. Peters, and H.C. Clevers. 2004. Complete polarization of single intestinal epithelial cells upon activation of LKB1 by STRAD. Cell. 116:457–466. - PubMed
-
- Bilder, D., M. Schober, and N. Perrimon. 2003. Integrated activity of PDZ protein complexes regulates epithelial polarity. Nat. Cell Biol. 5:53–58. - PubMed
-
- Bretscher, A., D. Chambers, R. Nguyen, and D. Reczek. 2000. ERM-Merlin and EBP50 protein families in plasma membrane organization and function. Annu. Rev. Cell Dev. Biol. 16:113–143. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

