Rapid hyperosmotic coinduction of two tilapia (Oreochromis mossambicus) transcription factors in gill cells
- PMID: 15642943
- PMCID: PMC545544
- DOI: 10.1073/pnas.0408956102
Rapid hyperosmotic coinduction of two tilapia (Oreochromis mossambicus) transcription factors in gill cells
Abstract
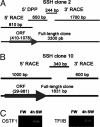
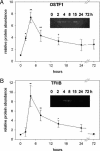
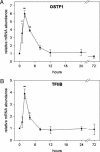
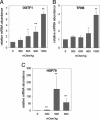
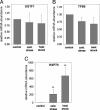
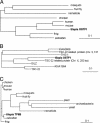
Gills of euryhaline teleosts are excellent models for studying osmotic-stress adaptation because they directly contact the aquatic environment and are an important effector tissue during osmotic stress. We acclimated tilapia (Oreochromis mossambicus) from fresh water (FW) to seawater (SW); performed suppression subtractive hybridization of gill mRNAs; and identified two transcription factors, osmotic stress transcription factor 1 (OSTF1) and the tilapia homolog of transcription factor II B (TFIIB), that are rapidly and transiently induced during hyperosmotic stress. mRNA levels increase 6-fold for OSTF1 and 4-fold for TFIIB, and they reach maxima 2 h after SW transfer. Protein levels increase 7.5-fold for OSTF1 and 9-fold for TFIIB, and they reach maxima 4 h after SW transfer. Induction of OSTF1 and TFIIB increases gradually with increasing salinity. Induction of OSTF1 and TFIIB is specific for osmotic stress and absent during oxidative stress (1 mM H2O2) or heat shock (+10 degrees C). Bioinformatic analysis of OSTF1 reveals that it is a transcription factor of the TGF-beta-stimulated clone 22/GILZ family. Because some mammalian homologs are strongly induced by glucocorticoids, OSTF1 may represent the molecular link between the SW hormone cortisol and transcriptional regulation of ion transport and cell differentiation in teleost gills. Coinduction of OSTF1 and TFIIB may serve to recruit TFIIB preferentially to OSTF1 target genes during hyperosmotic stress and compensate for reduced rates of transcription resulting from salt-induced chromatin compaction. We conclude that OSTF1 and TFIIB are critical elements of osmosensory signal transduction in euryhaline teleosts that mediate osmotic adaptation by means of transcriptional regulation.
Figures
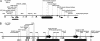
References
-
- Laurent, P. & Dunel, S. (1980) Am. J. Physiol. 238, R147-R159. - PubMed
-
- Evans, D. H. (2002) J. Exp. Zool. 293, 336-347. - PubMed
-
- Tipsmark, C. K., Madsen, S. S., Seidelin, M., Christensen, A. S., Cutler, C. P. & Cramb, G. (2002) J. Exp. Zool. 293, 106-118. - PubMed
-
- Mistry, A. C., Honda, S., Hirata, T., Kato, A. & Hirose, S. (2001) Am. J. Physiol. 281, R1594-R1604. - PubMed
-
- Cutler, C. P. & Cramb, G. (2002) J. Exp. Biol. 205, 2643-2651. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
LinkOut - more resources
Full Text Sources

