Acute inflammatory response to endotoxin in mice and humans
- PMID: 15642986
- PMCID: PMC540200
- DOI: 10.1128/CDLI.12.1.60-67.2005
Acute inflammatory response to endotoxin in mice and humans
Abstract
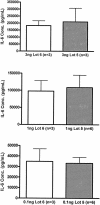
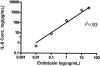
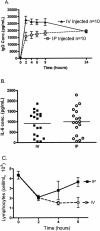
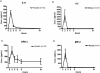
Endotoxin injection has been widely used to study the acute inflammatory response. In this study, we directly compared the inflammatory responses to endotoxin in mice and humans. Escherichia coli type O113 endotoxin was prepared under identical conditions, verified to be of equal biological potency, and used for both mice and humans. The dose of endotoxin needed to induce an interleukin-6 (IL-6) concentration in plasma of approximately 1,000 pg/ml 2 h after injection was 2 ng/kg of body weight in humans and 500 ng/kg in mice. Healthy adult volunteers were injected intravenously with endotoxin, and male C57BL/6 mice (n=4 to 12) were injected intraperitoneally with endotoxin. Physiological, hematological, and cytokine responses were determined. Endotoxin induced a rapid physiological response in humans (fever, tachycardia, and slight hypotension) but not in mice. Both mice and humans exhibited lymphopenia with a nadir at 4 h and recovery by 24 h. The levels of tumor necrosis factor (TNF) and IL-6 in plasma peaked at 2 h and returned to baseline levels by 4 to 6 h. IL-1 receptor antagonist RA and TNF soluble receptor I were upregulated in both mice and humans but were upregulated more strongly in humans. Mice produced greater levels of CXC chemokines, and both mice and humans exhibited peak production at 2 h. These studies demonstrate that although differences exist and a higher endotoxin challenge is necessary in mice, there are several similarities in the inflammatory response to endotoxin in mice and humans.
Figures



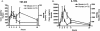
References
-
- Barber, A. E., S. M. Coyle, M. A. Marano, E. Fischer, S. E. Calvano, Y. Fong, L. L. Moldawer, and S. F. Lowry. 1993. Glucocorticoid therapy alters hormonal and cytokine responses to endotoxin in man. J. Immunol. 150:1999-2006. - PubMed
-
- Boujoukos, A. J., G. D. Martich, E. Supinski, and A. F. Suffredini. 1993. Compartmentalization of the acute cytokine response in humans after intravenous endotoxin administration. J. Appl. Physiol. 74:3027-3033. - PubMed
-
- Caldwell, F. T., Jr., D. B. Graves, and B. H. Wallace. 1999. Humoral versus neural pathways for fever production in rats after administration of lipopolysaccharide. J. Trauma 47:120-129. - PubMed
-
- Calvano, S. E., W. A. Thompson, M. N. Marra, S. M. Coyle, H. F. de Riesthal, R. K. Trousdale, P. S. Barie, R. W. Scott, L. L. Moldawer, and S. F. Lowry. 1994. Changes in polymorphonuclear leukocyte surface and plasma bactericidal/permeability-increasing protein and plasma lipopolysaccharide binding protein during endotoxemia or sepsis. Arch. Surg. 129:220-226. - PubMed
-
- Cannon, J. G., R. G. Tompkins, J. A. Gelfand, H. R. Michie, G. G. Stanford, J. W. van der Meer, S. Endres, G. Lonnemann, J. Corsetti, B. Chernow, et al. 1990. Circulating interleukin-1 and tumor necrosis factor in septic shock and experimental endotoxin fever. J. Infect. Dis. 161:79-84. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

