Coccidioides posadasii contains a single 1,3-beta-glucan synthase gene that appears to be essential for growth
- PMID: 15643067
- PMCID: PMC544152
- DOI: 10.1128/EC.4.1.111-120.2005
Coccidioides posadasii contains a single 1,3-beta-glucan synthase gene that appears to be essential for growth
Abstract
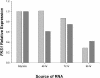
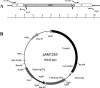
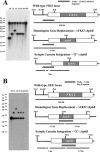
1,3-beta-Glucan synthase is responsible for the synthesis of beta-glucan, an essential cell wall structural component in most fungi. We sought to determine whether Coccidioides posadasii possesses genes homologous to known fungal FKS genes that encode the catalytic subunit of 1,3-beta-glucan synthase. A single gene, designated FKS1, was identified, and examination of its predicted protein product showed a high degree of conservation with Fks proteins from other filamentous fungi. FKS1 is expressed at similar levels in mycelia and early spherulating cultures, and expression decreases as the spherules mature. We used Agrobacterium-mediated transformation to create strains that harbor DeltaFKS1::hygB, a null allele of FKS1, and hypothesize that Fks1p function is essential, due to our inability to purify this allele away from a complementing wild-type FKS1 allele in a heterokaryotic strain. The heterokaryon appears normal with respect to growth rate and arthroconidium production; however, microscopic examination of strains with DeltaFKS1::hygB alleles revealed abnormal swelling of hyphal elements.
Figures


References
-
- Abuodeh, R. O., M. J. Orbach, M. A. Mandel, A. Das, and J. N. Galgiani. 2000. Genetic transformation of Coccidioides immitis facilitated by Agrobacterium tumefaciens. J. Infect. Dis. 181:2106-2110. - PubMed
-
- Bartizal, K., C. J. Gill, G. K. Abruzzo, A. M. Flattery, L. Kong, P. M. Scott, J. G. Smith, C. E. Leighton, A. Bouffard, J. F. Dropinski, and J. Balkovec. 1997. In vitro preclinical evaluation studies with the echinocandin antifungal MK-0991 (L-743,872). Antimicrob. Agents Chemother. 41:2326-2332. - PMC - PubMed
-
- Cabib, E., B. Bowers, A. Sburlati, and S. J. Silverman. 1988. Fungal cell wall synthesis: the construction of a biological structure. Microbiol. Sci. 5:370-375. - PubMed
-
- Carroll, A., J. Sweigard, and B. Valent. 1994. Improved vectors for selecting resistance to hygromycin. Fungal Genet. Newsl. 41:22.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

