Transcriptional network of multiple capsule and melanin genes governed by the Cryptococcus neoformans cyclic AMP cascade
- PMID: 15643074
- PMCID: PMC544166
- DOI: 10.1128/EC.4.1.190-201.2005
Transcriptional network of multiple capsule and melanin genes governed by the Cryptococcus neoformans cyclic AMP cascade
Abstract
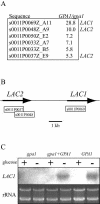
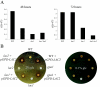
Cryptococcus neoformans is an opportunistic human fungal pathogen that elaborates several virulence attributes, including a polysaccharide capsule and melanin pigments. A conserved Galpha protein/cyclic AMP (cAMP) pathway controls melanin and capsule production. To identify targets of this pathway, we used an expression profiling approach to define genes that are transcriptionally regulated by the Galpha protein Gpa1. This approach revealed that Gpa1 transcriptionally regulates multiple genes involved in capsule assembly and identified two additional genes with a marked dependence on Gpa1 for transcription. The first is the LAC1 gene, encoding the laccase enzyme that catalyzes a rate-limiting step in diphenol oxidation and melanin production. The second gene identified (LAC2) is adjacent to the LAC1 gene and encodes a second laccase that shares 75% nucleotide identity with LAC1. Similar to the LAC1 gene, LAC2 is induced in response to glucose deprivation. However, LAC2 basal transcript levels are much lower than those for LAC1. Accordingly, a lac2 mutation results in only a modest delay in melanin formation. LAC2 overexpression suppresses the melanin defects of gpa1 and lac1 mutants and partially restores virulence of these strains. These studies provide mechanistic insights into the regulation of capsule and melanin production by the C. neoformans cAMP pathway and demonstrate that multiple laccases contribute to C. neoformans melanin production and pathogenesis.
Figures





References
-
- Alspaugh, J. A., R. Pukkila-Worley, T. Harashima, L. M. Cavallo, D. Funnell, G. M. Cox, J. R. Perfect, J. W. Kronstad, and J. Heitman. 2002. Adenylyl cyclase functions downstream of the G-alpha protein Gpa1 and controls mating and pathogenicity of Cryptococcus neoformans. Eukaryot. Cell 1:75-84. - PMC - PubMed
-
- Broach, J. R., and R. J. Deschenes. 1990. The functions of RAS genes in Saccharomyces cerevisiae. Adv. Cancer Res. 54:79-138. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

