Antimony-induced cardiomyopathy in guinea-pig and protection by L-carnitine
- PMID: 15644865
- PMCID: PMC1575978
- DOI: 10.1038/sj.bjp.0706030
Antimony-induced cardiomyopathy in guinea-pig and protection by L-carnitine
Abstract
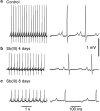
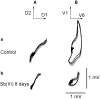
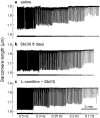
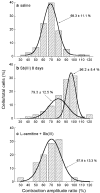
Antimony (Sb) is the mainstay for the treatment of Leishmaniasis. It has serious, often lethal, cardiovascular side effects. The objective of this study was to examine the effects of Sb treatment upon the electrocardiogram (ECG), myocyte contractility (assessed by monitoring sarcomere length during field stimulation), whole-cell action potential (AP) and calcium current (I(Ca)) of the guinea-pig and to evaluate L-carnitine as a cardioprotective agent. Guinea-pigs received daily injections of either saline, Sb(V), Sb(III), L-carnitine or L-carnitine with Sb(III). Eight lead ECGs were recorded under halothane anaesthesia every 4 days. At the end of each treatment regime, animals were killed and ventricular myocytes were enzymatically isolated. Treatment with Sb(V) for 26 days prolonged the QT interval of the ECG. Treatment with Sb(III) was lethal within 2 days for approximately 50% of the animals. The survivors showed ECG alterations similar to those described in man: T wave flattening and/or inversion, depression of the ST segment, and elongation of RR and QT intervals. Their ventricular myocytes showed impaired contraction responses to changes in stimulus frequency, elongated AP and reduced I(Ca). Combined treatment with L-carnitine and Sb(III) delayed mortality. Prior treatment with L-carnitine followed by combined treatment with L-carnitine and Sb(III) reduced mortality to <10% over 12 days and these animals showed normal ECG. Their myocytes showed normal contractility and AP. It is concluded that L-carnitine has a preventive cardioprotective role against antimony-induced cardiomyopathy. The mechanism of action of L-carnitine may be to counter oxidative stress caused by Sb(III).
Figures



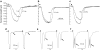
References
-
- BALSHAW D.M., XU L., YAMAGUCHI N., PASEK D.A., MEISSNER G. Calmodulin binding and inhibition of cardiac muscle calcium release channel (ryanodine receptor) J. Biol. Chem. 2001;276:20144–20153. - PubMed
-
- BERESEWICZ A., HORACKOVA M. Alterations in electrical and contractile behavior of isolated cardiomyocytes by hydrogen peroxide: possible ionic mechanisms. J. Mol. Cell. Cardiol. 1991;23:899–918. - PubMed
-
- BERS D.M. Excitation–Contraction Coupling and Cardiac Contractile Force 2001Boston: Kluwer Academic Press Publishers; 2nd edn.
-
- BORASO A., WILLIAMS A.J. Modification of the gating of the cardiac sarcoplasmic reticulum Ca(2+)-release channel by H2O2 and dithiothreitol. Am. J. Physiol. 1994;267:H1010–H1016. - PubMed
-
- BRADLEY W.R., FREDERICK W.G. The toxicity of antimony. Ind. Med. 1941;2:15–22.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

