Substituting abacavir for hyperlipidemia-associated protease inhibitors in HAART regimens improves fasting lipid profiles, maintains virologic suppression, and simplifies treatment
- PMID: 15647105
- PMCID: PMC548524
- DOI: 10.1186/1471-2334-5-2
Substituting abacavir for hyperlipidemia-associated protease inhibitors in HAART regimens improves fasting lipid profiles, maintains virologic suppression, and simplifies treatment
Abstract
Background: Hyperlipidemia secondary to protease inhibitors (PI) may abate by switching to anti-HIV medications without lipid effects.
Method: An open-label, randomized pilot study compared changes in fasting lipids and HIV-1 RNA in 104 HIV-infected adults with PI-associated hyperlipidemia (fasting serum total cholesterol >200 mg/dL) who were randomized either to a regimen in which their PI was replaced by abacavir 300 mg twice daily (n = 52) or a regimen in which their PI was continued (n = 52) for 28 weeks. All patients had undetectable viral loads (HIV-1 RNA <50 copies/mL) at baseline and were naive to abacavir and non-nucleoside reverse transcriptase inhibitors.
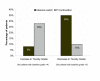
Results: At baseline, the mean total cholesterol was 243 mg/dL, low density lipoprotein (LDL)-cholesterol 149 mg/dL, high density lipoprotein (HDL)-cholesterol 41 mg/dL, and triglycerides 310 mg/dL. Mean CD4+ cell counts were 551 and 531 cells/mm3 in the abacavir-switch and PI-continuation arms, respectively. At week 28, the abacavir-switch arm had significantly greater least square mean reduction from baseline in total cholesterol (-42 vs -10 mg/dL, P < 0.001), LDL-cholesterol (-14 vs +5 mg/dL, P = 0.016), and triglycerides (-134 vs -36 mg/dL, P = 0.019) than the PI-continuation arm, with no differences in HDL-cholesterol (+0.2 vs +1.3 mg/dL, P = 0.583). A higher proportion of patients in the abacavir-switch arm had decreases in protocol-defined total cholesterol and triglyceride toxicity grades, whereas a smaller proportion had increases in these toxicity grades. At week 28, an intent-to treat: missing = failure analysis showed that the abacavir-switch and PI-continuation arms did not differ significantly with respect to proportion of patients maintaining HIV-1 RNA <400 or <50 copies/mL or adjusted mean change from baseline in CD4+ cell count. Two possible abacavir-related hypersensitivity reactions were reported. No significant changes in glucose, insulin, insulin resistance, C-peptide, or waist-to-hip ratios were observed in either treatment arm, nor were differences in these parameters noted between treatments.
Conclusion: In hyperlipidemic, antiretroviral-experienced patients with HIV-1 RNA levels <50 copies/mL and CD4+ cell counts >500 cells/mm3, substituting abacavir for hyperlipidemia-associated PIs in combination antiretroviral regimens improves lipid profiles and maintains virologic suppression over a 28-week period, and it simplifies treatment.
Figures




References
-
- Jordan J, Carranza Rosenzweig J, Pathak D, Pilon T. Perceived influence of regimen characteristics on adherence. 5th International Congress on Drug Therapy in HIV Infection Glasgow, United Kingdom. October 22–26, 2000.
-
- Currier JS. Cardiovascular risk associated with HIV therapy. J Acquir Immune Defic Syndr. 2002;31:S16–S23. - PubMed
-
- Periard D, Telenti A, Sudre P, Cheseaux JJ, Halfon P, Reymond MJ, Marcovina SM, Glauser MP, Nicod P, Darioli R, Mooser V. Atherogenic dyslipidemia in HIV-infected individuals treated with protease inhibitors. The Swiss HIV Cohort Study. Circulation. 1999;100:700–705. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

