Regulatory CD4+CD25+ cells reverse imbalances in the T cell pool of bone marrow transplanted TGepsilon26 mice leading to the prevention of colitis
- PMID: 15647183
- PMCID: PMC1774840
- DOI: 10.1136/gut.2004.046953
Regulatory CD4+CD25+ cells reverse imbalances in the T cell pool of bone marrow transplanted TGepsilon26 mice leading to the prevention of colitis
Abstract
Background and aims: Erroneous thymic selection of developing T lymphocytes may be responsible for the expansion of self reactive T cells or may contribute to the absence of regulatory T cells important in controlling peripheral inflammatory processes. Colitis in bone marrow (BM) transplanted Tgepsilon26 mice is induced by abnormally activated T cells developing in an aberrant thymic microenvironment. We investigated the protective role of regulatory CD4+CD25+ T cells in this model.
Methods: BM from (C57BL/6 x CBA/J) F1 mice was transplanted into specific pathogen free Tgepsilon26 mice (BM-->Tgepsilon26). Transplanted mice received no cells (control), sorted CD4+CD25+, or CD4+CD25- cells from mesenteric lymph nodes (MLN) of normal mice. MLN cell subsets were analysed using membrane markers. Cytokine secretion of MLN cells was measured using intracellular cytokine staining and cytokine secretion in anti-CD3 stimulated cell cultures. Colitis was measured by histological scores.
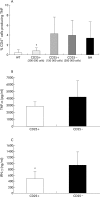
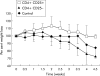
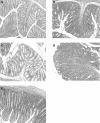
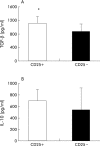
Results: CD4+CD25+ cells were reduced in the MLNs of BM-->Tgepsilon26 mice. Transfer of regulatory CD4CD4+CD25+ but not of CD4+CD25- cells reduced the number of MLN CD4+ T cells in BM-->Tgepsilon26 recipients and increased the number of MLN CD8+ cells, thereby normalising the CD4+/CD8+ ratio. CD4+CD25+ but not CD4+CD25- cell transfer into BM-->Tgepsilon26 mice reduced the number of tumour necrosis factor alpha+ CD4+ cells and increased the secretion of transforming growth factor beta by MLN cells. Transfer of 3 x 10(5) CD4+CD25+ cells after BM transplantation into Tgepsilon26 mice prevented colitis whereas CD4+CD25- cells had no protective effect.
Conclusions: These results suggest that defective selection or induction of regulatory T cells in the abnormal thymus is responsible for the development of colitis in BM-->Tgepsilon26 mice. Transfer of CD4+CD25+ cells can control intestinal inflammation in BM-->Tgepsilon26 mice by normalising the number and function of the MLN T cell pool.
Figures

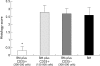
Similar articles
-
Regulatory CD4+ CD25+ T cells prevent thymic dysfunction in experimental chronic colitis.Scand J Immunol. 2007 Dec;66(6):636-44. doi: 10.1111/j.1365-3083.2007.02015.x. Scand J Immunol. 2007. PMID: 18021363
-
CD4+CD25+ cell depletion from the normal CD4+ T cell pool prevents tolerance toward the intestinal flora and leads to chronic colitis in immunodeficient mice.Inflamm Bowel Dis. 2006 Jun;12(6):437-46. doi: 10.1097/00054725-200606000-00002. Inflamm Bowel Dis. 2006. PMID: 16775487
-
Colitis is associated with thymic destruction attenuating CD4+25+ regulatory T cells in the periphery.Gastroenterology. 2004 Jun;126(7):1759-70. doi: 10.1053/j.gastro.2004.03.015. Gastroenterology. 2004. PMID: 15188171
-
Control of intestinal inflammation by regulatory T cells.Immunol Rev. 2001 Aug;182:190-200. doi: 10.1034/j.1600-065x.2001.1820115.x. Immunol Rev. 2001. PMID: 11722634 Review.
-
Regulatory T cells in experimental colitis.Curr Top Microbiol Immunol. 2005;293:179-208. doi: 10.1007/3-540-27702-1_9. Curr Top Microbiol Immunol. 2005. PMID: 15981481 Review.
Cited by
-
Inflammatory bowel disease: Moving toward a stem cell-based therapy.World J Gastroenterol. 2008 Aug 7;14(29):4616-26. doi: 10.3748/wjg.14.4616. World J Gastroenterol. 2008. PMID: 18698675 Free PMC article. Review.
-
Diminution of Circulating CD4+CD25 high T cells in naïve Crohn's disease.Dig Dis Sci. 2009 Oct;54(10):2084-93. doi: 10.1007/s10620-008-0590-6. Epub 2008 Dec 3. Dig Dis Sci. 2009. PMID: 19051021
-
Anti-inflammatory effects of Lactococcus lactis NCDO 2118 during the remission period of chemically induced colitis.Gut Pathog. 2014 Jul 29;6:33. doi: 10.1186/1757-4749-6-33. eCollection 2014. Gut Pathog. 2014. PMID: 25110521 Free PMC article.
References
-
- Garside P , Mowat AM. Oral tolerance. Semin Immunol 2001;13:177–85. - PubMed
-
- Sartor RB. Pathogenesis and immune mechanisms of chronic inflammatory bowel diseases. Am J Gastroenterol 1997;92:5S–11. - PubMed
-
- Strober W , Fuss IJ, Blumberg RS. The immunology of mucosal models of inflammation. Annu Rev Immunol 2002;20:495–549. - PubMed
-
- Mizoguchi A , Mizoguchi E, Bhan AK. Immune networks in animal models of inflammatory bowel disease. Inflamm Bowel Dis 2003;9:246–59. - PubMed
-
- Veltkamp C , Tonkonogy SL, De Yong YP, et al. Continuous stimulation by normal luminal bacteria is essential for the development and perpetuation of colitis in Tg26 mice. Gastroenterology 2001;120:900–13. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
