Requirement for serum response factor for skeletal muscle growth and maturation revealed by tissue-specific gene deletion in mice
- PMID: 15647354
- PMCID: PMC545866
- DOI: 10.1073/pnas.0409103102
Requirement for serum response factor for skeletal muscle growth and maturation revealed by tissue-specific gene deletion in mice
Abstract
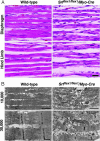
Serum response factor (SRF) controls the transcription of muscle genes by recruiting a variety of partner proteins, including members of the myocardin family of transcriptional coactivators. Mice lacking SRF fail to form mesoderm and die before gastrulation, precluding an analysis of the roles of SRF in muscle tissues. To investigate the functions of SRF in skeletal muscle development, we conditionally deleted the Srf gene in mice by skeletal muscle-specific expression of Cre recombinase. In mice lacking skeletal muscle SRF expression, muscle fibers formed, but failed to undergo hypertrophic growth after birth. Consequently, mutant mice died during the perinatal period from severe skeletal muscle hypoplasia. The myopathic phenotype of these mutant mice resembled that of mice expressing a dominant negative mutant of a myocardin family member in skeletal muscle. These findings reveal an essential role for the partnership of SRF and myocardin-related transcription factors in the control of skeletal muscle growth and maturation in vivo.
Figures




Similar articles
-
New role for serum response factor in postnatal skeletal muscle growth and regeneration via the interleukin 4 and insulin-like growth factor 1 pathways.Mol Cell Biol. 2006 Sep;26(17):6664-74. doi: 10.1128/MCB.00138-06. Mol Cell Biol. 2006. PMID: 16914747 Free PMC article.
-
Myocardin and ternary complex factors compete for SRF to control smooth muscle gene expression.Nature. 2004 Mar 11;428(6979):185-9. doi: 10.1038/nature02382. Nature. 2004. PMID: 15014501
-
Targeted inactivation of serum response factor in the developing heart results in myocardial defects and embryonic lethality.Mol Cell Biol. 2004 Jun;24(12):5281-9. doi: 10.1128/MCB.24.12.5281-5289.2004. Mol Cell Biol. 2004. PMID: 15169892 Free PMC article.
-
Control of smooth muscle development by the myocardin family of transcriptional coactivators.Curr Opin Genet Dev. 2004 Oct;14(5):558-66. doi: 10.1016/j.gde.2004.08.003. Curr Opin Genet Dev. 2004. PMID: 15380248 Review.
-
The myocardin family of transcriptional coactivators: versatile regulators of cell growth, migration, and myogenesis.Genes Dev. 2006 Jun 15;20(12):1545-56. doi: 10.1101/gad.1428006. Genes Dev. 2006. PMID: 16778073 Review.
Cited by
-
Suppressing PDGFRβ Signaling Enhances Myocyte Fusion to Promote Skeletal Muscle Regeneration.bioRxiv [Preprint]. 2024 Oct 18:2024.10.15.618247. doi: 10.1101/2024.10.15.618247. bioRxiv. 2024. PMID: 39464006 Free PMC article. Preprint.
-
A mouse model of rhabdomyosarcoma originating from the adipocyte lineage.Cancer Cell. 2012 Oct 16;22(4):536-46. doi: 10.1016/j.ccr.2012.09.004. Cancer Cell. 2012. PMID: 23079662 Free PMC article.
-
Serum Response Factor in Muscle Tissues: From Development to Ageing.Eur J Transl Myol. 2016 Jun 22;26(2):6008. doi: 10.4081/ejtm.2016.6008. eCollection 2016 Jun 13. Eur J Transl Myol. 2016. PMID: 27478561 Free PMC article.
-
Gene knockout of Acc2 has little effect on body weight, fat mass, or food intake.Proc Natl Acad Sci U S A. 2010 Apr 20;107(16):7598-603. doi: 10.1073/pnas.0913492107. Epub 2010 Apr 5. Proc Natl Acad Sci U S A. 2010. PMID: 20368432 Free PMC article.
-
Enzymatically crosslinked gelatin-laminin hydrogels for applications in neuromuscular tissue engineering.Biomater Sci. 2020 Jan 21;8(2):591-606. doi: 10.1039/c9bm01430f. Biomater Sci. 2020. PMID: 31859298 Free PMC article.
References
-
- Molkentin, J. D., Black, B. L., Martin, J. F. & Olson, E. N. (1995) Cell 83, 1125–1136. - PubMed
-
- Buckingham, M. (2001) Curr. Opin. Genet. Dev. 11, 440–448. - PubMed
-
- Pownall, M. E., Gustafsson, M. K. & Emerson, C. P., Jr. (2002) Annu. Rev. Cell Dev. Biol. 18, 747–783. - PubMed
-
- Norman, C., Runswick, M., Pollock, R. & Treisman, R. (1988) Cell 55, 989–1003. - PubMed
-
- Miano, J. M. (2003) J. Mol. Cell Cardiol. 35, 577–593. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

