Src-dependent ezrin phosphorylation in adhesion-mediated signaling
- PMID: 15647376
- PMCID: PMC551509
- DOI: 10.1091/mbc.e04-08-0721
Src-dependent ezrin phosphorylation in adhesion-mediated signaling
Abstract
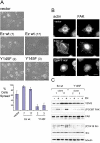
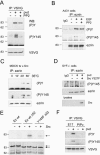
In addition to providing a regulated linkage between the membrane and the actin cytoskeleton, ezrin participates in signal transduction pathways. Here we describe that expression of the ezrin Y145F mutant delays epithelial cell spreading on fibronectin by inhibiting events leading to FAK activation. The defect in spreading was rescued by the overexpression of catalytically functional Src. We demonstrate that ezrin Y145 is phosphorylated in A431 cells stimulated with epidermal growth factor (EGF) and in v-Src-transformed cells. Moreover in cells devoid of Src, SYF-/- fibroblasts, ezrin Y145 phosphorylation could only be detected upon the introduction of an active form of Src. The phosphorylation of ezrin at Y145 required prior binding of the Src SH2 domain to ezrin. Our results further show that Src activity influences its binding to ezrin and a positive feedback mechanism for Src-mediated Y145 phosphorylation is implied. Interestingly, cells expressing ezrin Y145F did not proliferate when cultured in a 3D collagen gel. Collectively, our results demonstrate a key signaling input of Src-dependent ezrin phosphorylation in adhesion-mediated events in epithelial cells.
Figures





References
-
- Autero, M., Heiska, L., Ronnstrand, L., Vaheri, A., Gahmberg, C. G., and Carpen, O. (2003). Ezrin is a substrate for Lck in T cells. FEBS Lett. 535, 82-86. - PubMed
-
- Behrens, J., Vakaet, L., Friis, R., Winterhager, E., Van Roy, F., Mareel, M. M., and Birchmeier, W. (1993). Loss of epithelial differentiation and gain of invasiveness correlates with tyrosine phosphorylation of the E-cadherin/beta-catenin complex in cells transformed with a temperature-sensitive v-SRC gene. J. Cell Biol. 120, 757-766. - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

