Defining the properties of the nonhelical tail domain in type II keratin 5: insight from a bullous disease-causing mutation
- PMID: 15647384
- PMCID: PMC551504
- DOI: 10.1091/mbc.e04-06-0498
Defining the properties of the nonhelical tail domain in type II keratin 5: insight from a bullous disease-causing mutation
Abstract
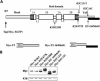
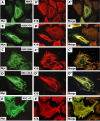
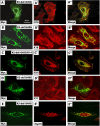
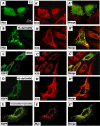
Inherited mutations in the intermediate filament (IF) proteins keratin 5 (K5) or keratin 14 (K14) cause epidermolysis bullosa simplex (EBS), in which basal layer keratinocytes rupture upon trauma to the epidermis. Most mutations are missense alleles affecting amino acids located in the central alpha-helical rod domain of K5 and K14. Here, we study the properties of an unusual EBS-causing mutation in which a nucleotide deletion (1649delG) alters the last 41 amino acids and adds 35 residues to the C terminus of K5. Relative to wild type, filaments coassembled in vitro from purified K5-1649delG and K14 proteins are shorter and exhibit weak viscoelastic properties when placed under strain. Loss of the C-terminal 41 residues contributes to these alterations. When transfected in cultured epithelial cells, K5-1649delG incorporates into preexisting keratin IFs and also forms multiple small aggregates that often colocalize with hsp70 in the cytoplasm. Aggregation is purely a function of the K5-1649delG tail domain; in contrast, the cloned 109 residue-long tail domain from wild type K5 is distributed throughout the cytoplasm and colocalizes partly with keratin IFs. These data provide a mechanistic basis for the cell fragility seen in individuals bearing the K5-1649delG allele, and point to the role of the C-terminal 41 residues in determining K5's assembly properties.
Figures

Similar articles
-
Interaction of plectin with keratins 5 and 14: dependence on several plectin domains and keratin quaternary structure.J Invest Dermatol. 2014 Nov;134(11):2776-2783. doi: 10.1038/jid.2014.255. Epub 2014 Jun 18. J Invest Dermatol. 2014. PMID: 24940650
-
Complete cytolysis and neonatal lethality in keratin 5 knockout mice reveal its fundamental role in skin integrity and in epidermolysis bullosa simplex.Mol Biol Cell. 2001 Jun;12(6):1775-89. doi: 10.1091/mbc.12.6.1775. Mol Biol Cell. 2001. PMID: 11408584 Free PMC article.
-
Molecular origin of the effects of mutation on the structure and mechanical properties of human epithelial keratin K5/K14.J Mech Behav Biomed Mater. 2021 Dec;124:104798. doi: 10.1016/j.jmbbm.2021.104798. Epub 2021 Sep 2. J Mech Behav Biomed Mater. 2021. PMID: 34509171
-
Defining keratin protein function in skin epithelia: epidermolysis bullosa simplex and its aftermath.J Invest Dermatol. 2012 Mar;132(3 Pt 2):763-75. doi: 10.1038/jid.2011.450. Epub 2012 Jan 26. J Invest Dermatol. 2012. PMID: 22277943 Free PMC article. Review.
-
Human keratin diseases: hereditary fragility of specific epithelial tissues.Exp Dermatol. 1996 Dec;5(6):297-307. doi: 10.1111/j.1600-0625.1996.tb00133.x. Exp Dermatol. 1996. PMID: 9028791 Review.
Cited by
-
Functional Analysis of Keratin-Associated Proteins in Intestinal Epithelia: Heat-Shock Protein Chaperoning and Kinase Rescue.Methods Enzymol. 2016;569:139-54. doi: 10.1016/bs.mie.2015.08.019. Epub 2015 Sep 8. Methods Enzymol. 2016. PMID: 26778557 Free PMC article.
-
Epidermolysis bullosa simplex: a paradigm for disorders of tissue fragility.J Clin Invest. 2009 Jul;119(7):1784-93. doi: 10.1172/JCI38177. Epub 2009 Jul 1. J Clin Invest. 2009. PMID: 19587453 Free PMC article. Review.
-
Hedgehog signaling, keratin 6 induction, and sebaceous gland morphogenesis: implications for pachyonychia congenita and related conditions.Am J Pathol. 2008 Sep;173(3):752-61. doi: 10.2353/ajpath.2008.071089. Epub 2008 Aug 7. Am J Pathol. 2008. PMID: 18688029 Free PMC article.
-
Multiple roles for keratin intermediate filaments in the regulation of epithelial barrier function and apico-basal polarity.Tissue Barriers. 2016 May 2;4(3):e1178368. doi: 10.1080/21688370.2016.1178368. eCollection 2016 Jul-Sep. Tissue Barriers. 2016. PMID: 27583190 Free PMC article. Review.
-
Loose Anagen Hair Associated with Wooly Hair Caused by a Heterozygous, Intronic KRT71 Variant.Genes (Basel). 2025 Apr 17;16(4):459. doi: 10.3390/genes16040459. Genes (Basel). 2025. PMID: 40282419 Free PMC article.
References
-
- Anton-Lamprecht, I. (1983). Genetically induced abnormalities of epidermal differentiation and ultrastructure in ichthyoses and epidermolyses: pathogenesis, heterogeneity, fetal manifestation, and prenatal diagnosis. J. Investig. Dermatol 81, 149s-156s. - PubMed
-
- Bonifas, J. M., Rothman, A. L., Epstein, E. H., Jr. (1991). Epidermolysis bullosa simplex: evidence in two families for keratin gene abnormalities. Science 254, 1202-1205. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

