The micromolar zinc-binding domain on the NMDA receptor subunit NR2B
- PMID: 15647474
- PMCID: PMC6725474
- DOI: 10.1523/JNEUROSCI.3967-04.2005
The micromolar zinc-binding domain on the NMDA receptor subunit NR2B
Abstract
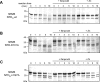
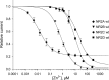
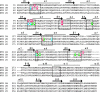
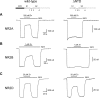
Eukaryotic ionotropic glutamate receptor subunits possess a large N-terminal domain (NTD) distinct from the neighboring agonist-binding domain. In NMDA receptors, the NTDs of NR2A and NR2B form modulatory domains binding allosteric inhibitors. Despite a high sequence homology, these two domains have been shown to bind two ligands of strikingly different chemical nature. Whereas the NTD of NR2A binds zinc with high (nanomolar) affinity, the NTD of NR2B binds the synthetic neuroprotectant ifenprodil and its derivatives. Using both NTD-mutated/deleted receptors and isolated NTDs, we now show that the NTD of NR2B, in contrast to NR2C and NR2D, also binds zinc, but with a lower affinity. Furthermore, we present evidence that zinc and ifenprodil compete for an overlapping binding site. This modulatory binding site accounts for the submicromolar zinc inhibition of NR1/NR2B receptors. Given that zinc is accumulated and released at many glutamatergic synapses in the CNS, these findings suggest that zinc is the endogenous ligand of the NTD of both NR2A and NR2B, the two major NR2 subunits. Thus, NMDA receptors contain zinc sensors capable of detecting extracellular zinc over a wide concentration range depending on their NR2 subunit composition. The coexistence of subunit-specific zinc-binding sites of high (nanomolar) and low (micromolar) affinity on NMDA receptors raises the possibility that zinc exerts both a tonic and a phasic control of membrane excitability.
Figures



References
-
- Armstrong N, Sun Y, Chen GQ, Gouaux E (1998) Structure of a glutamate-receptor ligand-binding core in complex with kainate. Nature 395: 913-917. - PubMed
-
- Ayalon G, Stern-Bach Y (2001) Functional assembly of AMPA and kainate receptors is mediated by several discrete protein-protein interactions. Neuron 31: 103-113. - PubMed
-
- Bogden JD, Troiano RA, Joselow MM (1977) Copper, zinc, magnesium, and calcium in plasma and cerebrospinal fluid of patients with neurological diseases. Clin Chem 23: 485-489. - PubMed
-
- Chen GQ, Cui C, Mayer ML, Gouaux E (1999) Functional characterization of a potassium-selective prokaryotic glutamate receptor. Nature 402: 817-821. - PubMed
-
- Chen N, Moshaver A, Raymond LA (1997) Differential sensitivity of recombinant N-methyl-d-aspartate receptor subtypes to zinc inhibition. Mol Pharmacol 51: 1015-1023. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
