Longer forms of amyloid beta protein: implications for the mechanism of intramembrane cleavage by gamma-secretase
- PMID: 15647487
- PMCID: PMC6725472
- DOI: 10.1523/JNEUROSCI.1575-04.2005
Longer forms of amyloid beta protein: implications for the mechanism of intramembrane cleavage by gamma-secretase
Abstract
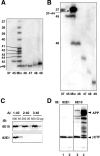
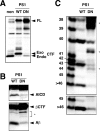
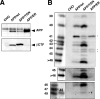
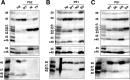
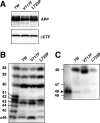
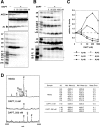
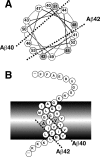
Gamma-cleavage of beta-amyloid precursor protein (APP) in the middle of the cell membrane generates amyloid beta protein (Abeta), and epsilon-cleavage, approximately 10 residues downstream of the gamma-cleavage site, releases the APP intracellular domain (AICD). A significant link between generation of Abeta and AICD and failure to detect AICD41-99 led us to hypothesize that epsilon-cleavage generates longer Abetas, which are then processed to Abeta40/42. Using newly developed gel systems and an N-end-specific monoclonal antibody, we have identified the longer Abetas (Abeta1-43, Abeta1-45, Abeta1-46, and Abeta1-48) within the cells and in brain tissues. The production of these longer Abetas as well as Abeta40/42 is presenilin dependent and is suppressed by {1S-benzyl-4R-[1S-carbamoyl-2-phenylethylcarbamoyl-1S-3-methylbutylcarbamoyl]-2R-hydroxy-5-phenylpentyl}carbamic acid tert-butyl ester, a transition state analog inhibitor for aspartyl protease. In contrast, N-[N-(3,5-difluorophenacetyl)-L-alanyl]-S-phenylglycine t-butyl ester, a potent dipeptide gamma-secretase inhibitor, builds up Abeta1-43 and Abeta1-46 intracellularly, which was also confirmed by mass spectrometry. Notably, suppression of Abeta40 appeared to lead to an increase in Abeta43, which in turn brings an increase in Abeta46, in a dose-dependent manner. We therefore propose an alpha-helical model in which longer Abeta species generated by epsilon-cleavage is cleaved at every three residues in its carboxyl portion.
Figures


References
-
- Beher D, Wrigley JD, Owens AP, Shearman MS (2002) Generation of C-terminally truncated amyloid-β peptides is dependent on γ-secretase activity. J Neurochem 82: 563-575. - PubMed
-
- Citron M, Oltersdorf T, Haass C, McConlogue L, Hung AY, Seubert P, VigoPelfrey C, Lieberburg I, Selkoe DJ (1992) Mutation of the β-amyloid precursor protein in familial Alzheimer's disease increases β-protein production. Nature 360: 672-674. - PubMed
-
- Clarke NJ, Tomlinson AJ, Ohyagi Y, Younkin S, Naylor S (1998) Detection and quantitation of cellularly derived amyloid β peptides by immunoprecipitation-HPLC-MS. FEBS Lett 430: 419-423. - PubMed
-
- De Strooper B, Saftig P, Craessaerts K, Vanderstichele H, Guhde G, Annaert W, Von Figura K, Van Leuven F (1998) Deficiency of presenilin-1 inhibits the normal cleavage of amyloid precursor protein. Nature 391: 387-390. - PubMed
-
- Dovey HF, John V, Anderson JP, Chen LZ, de Saint Andrieu P, Fang LY, Freedman SB, Folmer B, Goldbach E, Holsztynska EJ, Hu KL, Johnson-Wood KL, Kennedy SL, Kholodenko D, Knops JE, Latimer LH, Lee M, Liao Z, Lievergurg IM, Motter RN, et al. (2001) Functional γ-secretase inhibitors reduce β-amyloid peptide levels in brain. J Neurochem 76: 173-181. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
