Sodium and calcium current-mediated pacemaker neurons and respiratory rhythm generation
- PMID: 15647488
- PMCID: PMC6725489
- DOI: 10.1523/JNEUROSCI.2237-04.2005
Sodium and calcium current-mediated pacemaker neurons and respiratory rhythm generation
Abstract
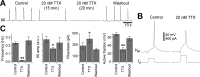
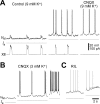
The breathing motor pattern in mammals originates in brainstem networks. Whether pacemaker neurons play an obligatory role remains a key unanswered question. We performed whole-cell recordings in the preBotzinger Complex in slice preparations from neonatal rodents and tested for pacemaker activity. We observed persistent Na+ current (I(NaP))-mediated bursting in approximately 5% of inspiratory neurons in postnatal day 0 (P0)-P5 and in P8-P10 slices. I(NaP)-mediated bursting was voltage dependent and blocked by 20 mum riluzole (RIL). We found Ca2+ current (I(Ca))-dependent bursting in 7.5% of inspiratory neurons in P8-P10 slices, but in P0-P5 slices these cells were exceedingly rare (0.6%). This bursting was voltage independent and blocked by 100 microm Cd2+ or flufenamic acid (FFA) (10-200 microm), which suggests that a Ca2+-activated inward cationic current (I(CAN)) underlies burst generation. These data substantiate our observation that P0-P5 slices exposed to RIL contain few (if any) pacemaker neurons, yet maintain respiratory rhythm. We also show that 20 nm TTX or coapplication of 20 microm RIL + FFA (100-200 microm) stops the respiratory rhythm, but that adding 2 mum substance P restarts it. We conclude that I(NaP) and I(CAN) enhance neuronal excitability and promote rhythmogenesis, even if their magnitude is insufficient to support bursting-pacemaker activity in individual neurons. When I(NaP) and I(CAN) are removed pharmacologically, the rhythm can be maintained by boosting neural excitability, which is inconsistent with a pacemaker-essential mechanism of respiratory rhythmogenesis by the preBotzinger complex.
Figures






Similar articles
-
Persistent sodium current, membrane properties and bursting behavior of pre-bötzinger complex inspiratory neurons in vitro.J Neurophysiol. 2002 Nov;88(5):2242-50. doi: 10.1152/jn.00081.2002. J Neurophysiol. 2002. PMID: 12424266
-
Role of neurokinin receptors and ionic mechanisms within the respiratory network of the lamprey.Neuroscience. 2010 Sep 1;169(3):1136-49. doi: 10.1016/j.neuroscience.2010.06.004. Epub 2010 Jun 9. Neuroscience. 2010. PMID: 20540991
-
Role of persistent sodium current in mouse preBötzinger Complex neurons and respiratory rhythm generation.J Physiol. 2007 Apr 15;580(Pt. 2):485-96. doi: 10.1113/jphysiol.2006.124602. Epub 2007 Feb 1. J Physiol. 2007. PMID: 17272351 Free PMC article.
-
PreBötzinger complex and pacemaker neurons: hypothesized site and kernel for respiratory rhythm generation.Annu Rev Physiol. 1998;60:385-405. doi: 10.1146/annurev.physiol.60.1.385. Annu Rev Physiol. 1998. PMID: 9558470 Review.
-
Neuronal mechanisms of respiratory rhythm generation: an approach using in vitro preparation.Jpn J Physiol. 1997 Oct;47(5):385-403. doi: 10.2170/jjphysiol.47.385. Jpn J Physiol. 1997. PMID: 9504127 Review.
Cited by
-
Prostaglandin E2 differentially modulates the central control of eupnoea, sighs and gasping in mice.J Physiol. 2015 Jan 1;593(1):305-19. doi: 10.1113/jphysiol.2014.279794. Epub 2014 Nov 3. J Physiol. 2015. PMID: 25556802 Free PMC article.
-
Understanding the rhythm of breathing: so near, yet so far.Annu Rev Physiol. 2013;75:423-52. doi: 10.1146/annurev-physiol-040510-130049. Epub 2012 Oct 29. Annu Rev Physiol. 2013. PMID: 23121137 Free PMC article. Review.
-
Neuronal mechanisms of respiratory pattern generation are evolutionary conserved.J Neurosci. 2013 May 22;33(21):9104-12. doi: 10.1523/JNEUROSCI.0299-13.2013. J Neurosci. 2013. PMID: 23699521 Free PMC article.
-
Effects of persistent sodium current blockade in respiratory circuits depend on the pharmacological mechanism of action and network dynamics.PLoS Comput Biol. 2019 Aug 30;15(8):e1006938. doi: 10.1371/journal.pcbi.1006938. eCollection 2019 Aug. PLoS Comput Biol. 2019. PMID: 31469828 Free PMC article.
-
Preparing for the first breath: prenatal maturation of respiratory neural control.J Physiol. 2006 Feb 1;570(Pt 3):437-44. doi: 10.1113/jphysiol.2005.097238. Epub 2005 Nov 10. J Physiol. 2006. PMID: 16284077 Free PMC article. Review.
References
-
- Ballanyi K, Onimaru H, Homma I (1999) Respiratory network function in the isolated brainstem-spinal cord of newborn rats. Prog Neurobiol 59: 583-634. - PubMed
-
- Ballerini L, Bracci E, Nistri A (1997) Pharmacological block of the electrogenic sodium pump disrupts rhythmic bursting induced by strychnine and bicuculline in the neonatal rat spinal cord. J Neurophysiol 77: 17-23. - PubMed
-
- Butera Jr RJ, Rinzel J, Smith JC (1999) Models of respiratory rhythm generation in the pre-Bötzinger complex. II. Populations of coupled pacemaker neurons. J Neurophysiol 82: 398-415. - PubMed
-
- Darbon P, Scicluna L, Tscherter A, Streit J (2002) Mechanisms controlling bursting activity induced by disinhibition in spinal cord networks. Eur J Neurosci 15: 671-683. - PubMed
-
- Darbon P, Tscherter A, Yvon C, Streit J (2003) Role of the electrogenic Na/K pump in disinhibition-induced bursting in cultured spinal networks. J Neurophysiol 90: 3119-3129. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
