Central glucocorticoid receptors modulate the expression and function of spinal NMDA receptors after peripheral nerve injury
- PMID: 15647493
- PMCID: PMC6725479
- DOI: 10.1523/JNEUROSCI.4127-04.2005
Central glucocorticoid receptors modulate the expression and function of spinal NMDA receptors after peripheral nerve injury
Abstract
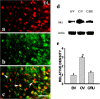
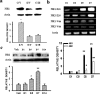
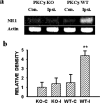
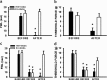
Central glucocorticoid receptors (GRs) and NMDA receptors (NMDARs) have been shown to play a significant role in the mechanisms of neuropathic pain after peripheral nerve injury; however, how central GRs and NMDARs interact in this process remains unknown. Here we show that the expression and function of spinal NMDARs after peripheral nerve injury were modulated by central GRs. Chronic constriction nerve injury (CCI) in rats induced a time-dependent upregulation of NR1 and NR2 subunits of the NMDAR within the spinal cord dorsal horn ipsilateral to CCI. The upregulation of NMDARs was significantly diminished by intrathecal administration (twice daily for postoperative days 1-6) of either the GR antagonist RU38486 or an antisense oligonucleotide against GRs. Moreover, this CCI-induced expression of NMDARs was significantly attenuated in rats receiving intrathecal treatment with an interleukin-6 (IL-6) antiserum and in mice with protein kinase Cgamma (PKCgamma) knock-out. Because IL-6 and PKCgamma mediated the upregulation of central GRs after CCI as demonstrated previously, the results suggest that IL-6 and PKCgamma served as cellular mediators contributing to the GR-mediated expression of NMDARs after CCI. Functionally, nociceptive behaviors induced by NMDAR activation and CCI were reversed by a single intrathecal administration of the GR antagonist RU38486. Conversely, a single intrathecal injection with the noncompetitive NMDAR antagonist MK-801 reversed neuropathic pain behaviors exacerbated by the GR agonist dexamethasone in CCI rats. These data suggest that interactions between central GRs and NMDARs through genomic and nongenomic regulation may be an important mechanism critical to neuropathic pain behaviors in rats.
Figures



Similar articles
-
Expression of central glucocorticoid receptors after peripheral nerve injury contributes to neuropathic pain behaviors in rats.J Neurosci. 2004 Sep 29;24(39):8595-605. doi: 10.1523/JNEUROSCI.3058-04.2004. J Neurosci. 2004. PMID: 15456833 Free PMC article.
-
Expression of spinal NMDA receptor and PKCgamma after chronic morphine is regulated by spinal glucocorticoid receptor.J Neurosci. 2005 Nov 30;25(48):11145-54. doi: 10.1523/JNEUROSCI.3768-05.2005. J Neurosci. 2005. PMID: 16319314 Free PMC article.
-
Central glucocorticoid receptors regulate the upregulation of spinal cannabinoid-1 receptors after peripheral nerve injury in rats.Pain. 2007 Sep;131(1-2):96-105. doi: 10.1016/j.pain.2006.12.019. Epub 2007 Jan 26. Pain. 2007. PMID: 17258396
-
Presynaptic NMDA receptors control nociceptive transmission at the spinal cord level in neuropathic pain.Cell Mol Life Sci. 2019 May;76(10):1889-1899. doi: 10.1007/s00018-019-03047-y. Epub 2019 Feb 20. Cell Mol Life Sci. 2019. PMID: 30788514 Free PMC article. Review.
-
The participation of galanin in pain processing at the spinal level.Trends Pharmacol Sci. 2002 Oct;23(10):468-74. doi: 10.1016/s0165-6147(02)02074-6. Trends Pharmacol Sci. 2002. PMID: 12368071 Review.
Cited by
-
Analgesic effects of ASP3662, a novel 11β-hydroxysteroid dehydrogenase 1 inhibitor, in rat models of neuropathic and dysfunctional pain.Br J Pharmacol. 2018 Oct;175(19):3784-3796. doi: 10.1111/bph.14448. Epub 2018 Aug 17. Br J Pharmacol. 2018. PMID: 30006998 Free PMC article.
-
Early dexamethasone treatment after implantation of a sciatic-nerve cuff decreases the concentration of substance P in the lumbar spinal cord of rats with neuropathic pain.Can J Vet Res. 2007 Apr;71(2):90-7. Can J Vet Res. 2007. PMID: 17479771 Free PMC article.
-
Preoperative anxiety-induced glucocorticoid signaling reduces GABAergic markers in spinal cord and promotes postoperative hyperalgesia by affecting neuronal PAS domain protein 4.Mol Pain. 2019 Jan-Dec;15:1744806919850383. doi: 10.1177/1744806919850383. Mol Pain. 2019. PMID: 31041873 Free PMC article.
-
Overlapping signatures of chronic pain in the DNA methylation landscape of prefrontal cortex and peripheral T cells.Sci Rep. 2016 Jan 28;6:19615. doi: 10.1038/srep19615. Sci Rep. 2016. PMID: 26817950 Free PMC article.
-
Inhibiting spinal secretory phospholipase A2 after painful nerve root injury attenuates established pain and spinal neuronal hyperexcitability by altering spinal glutamatergic signaling.Mol Pain. 2021 Jan-Dec;17:17448069211066221. doi: 10.1177/17448069211066221. Mol Pain. 2021. PMID: 34919471 Free PMC article.
References
-
- Abraham I, Harkany T, Horvath K, Veenema AH, Penke B, Nyakas C, Luiten PG (2000) Chronic corticosterone administration dose-dependently modulates abeta(1-42)- and NMDA-induced neurodegeneration in rat magnocellular nucleus basalis. J Neuroendocrinol 12: 486-494. - PubMed
-
- Arruda JL, Colburn RW, Rickman AJ, Rutkoski MD, Deleo JA (1998) Increase of interleukin-6 mRNA in the spinal cord following peripheral injury in the rat: potential role of IL-6 in neuropathic pain. Brain Res Mol Brain Res 62: 228-235. - PubMed
-
- Barkhudaryan N, Dunn AJ (1999) Molecular mechanisms of actions of interleukin-6 on the brain, with special reference to serotonin and the hypothalamo-pituitary-adrenocortical axis. Neurochem Res 24: 1169-1180. - PubMed
-
- Barrett TJ, Vedeckis WV (1996) Occupancy and composition of proteins bound to the AP-1 sites in the glucocorticoid receptor and c-jun promoters after glucocorticoid treatment and in different cell types. Recept Signal Transduct 6: 179-193. - PubMed
-
- Bennett GJ, Xie YK (1988) A peripheral mononeuropathy in rat that produces disorders of pain sensation like those seen in man. Pain 33: 87-107. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
