Ketamine induces dopamine-dependent depression of evoked hippocampal activity in the nucleus accumbens in freely moving rats
- PMID: 15647498
- PMCID: PMC6725480
- DOI: 10.1523/JNEUROSCI.3800-04.2005
Ketamine induces dopamine-dependent depression of evoked hippocampal activity in the nucleus accumbens in freely moving rats
Abstract
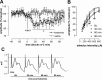
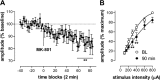
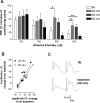
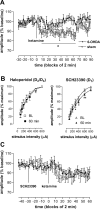
Noncompetitive NMDA receptor antagonists, such as ketamine, induce a transient schizophrenia-like state in healthy individuals and exacerbate psychosis in schizophrenic patients. In rodents, noncompetitive NMDA receptor antagonists induce a behavioral syndrome that represents an experimentally valid model of schizophrenia. Current experimental evidence has implicated the nucleus accumbens in the pathophysiology of schizophrenia and the psychomimetic actions of ketamine. In this study, we have demonstrated that acute systemic administration of ketamine, at a dose known to produce hyperlocomotion and stereotypy, depressed the amplitude of the monosynaptic component of fimbria-evoked field potentials recorded in the nucleus accumbens. A similar effect was observed using the more selective antagonist dizocilpine maleate, indicating the depression was NMDA receptor dependent. Paired-pulse facilitation was enhanced concomitantly with, and in proportion to, ketamine-induced depressed synaptic efficacy, indicative of a presynaptic mechanism of action. Notably, the depression of field potentials recorded in the nucleus accumbens was markedly reduced after a focal 6-hydroxydopamine lesioning procedure in the nucleus accumbens. More specifically, pretreatment with the D2/D4 antagonist haloperidol, but not the D1 antagonist SCH23390 blocked ketamine-induced depression of nucleus accumbens responses. Our findings provide supporting evidence for the contemporary theory of schizophrenia as aberrant excitatory neurotransmission at the level of the nucleus accumbens.
Figures

Similar articles
-
Responses of the nucleus accumbens following fornix/fimbria stimulation in the rat. Identification and long-term potentiation of mono- and polysynaptic pathways.Neuroscience. 1993 Apr;53(4):1049-58. doi: 10.1016/0306-4522(93)90488-2. Neuroscience. 1993. PMID: 8389427
-
Modulation of hippocampal and amygdalar-evoked activity of nucleus accumbens neurons by dopamine: cellular mechanisms of input selection.J Neurosci. 2001 Apr 15;21(8):2851-60. doi: 10.1523/JNEUROSCI.21-08-02851.2001. J Neurosci. 2001. PMID: 11306637 Free PMC article.
-
Ketamine, at a dose that disrupts motor behavior and latent inhibition, enhances prefrontal cortex synaptic efficacy and glutamate release in the nucleus accumbens.Neuropsychopharmacology. 2007 Mar;32(3):719-27. doi: 10.1038/sj.npp.1301057. Epub 2006 Mar 8. Neuropsychopharmacology. 2007. PMID: 16525415
-
[Glutaminergic hypothesis of schizophrenia: clinical research studies with ketamine].Encephale. 2001 Jan-Feb;27(1):53-9. Encephale. 2001. PMID: 11294039 Review. French.
-
Long-lasting changes in hippocampal synaptic plasticity and cognition in an animal model of NMDA receptor dysfunction in psychosis.Neuropharmacology. 2013 Nov;74:48-58. doi: 10.1016/j.neuropharm.2013.01.001. Epub 2013 Jan 31. Neuropharmacology. 2013. PMID: 23376021 Review.
Cited by
-
Acute ketamine induces hippocampal synaptic depression and spatial memory impairment through dopamine D1/D5 receptors.Psychopharmacology (Berl). 2013 Aug;228(3):451-61. doi: 10.1007/s00213-013-3048-2. Epub 2013 Mar 14. Psychopharmacology (Berl). 2013. PMID: 23494232
-
The bivalent side of the nucleus accumbens.Neuroimage. 2009 Feb 1;44(3):1178-87. doi: 10.1016/j.neuroimage.2008.09.039. Epub 2008 Oct 11. Neuroimage. 2009. PMID: 18976715 Free PMC article.
-
Repeated Nitrous Oxide Exposure Exerts Antidepressant-Like Effects Through Neuronal Nitric Oxide Synthase Activation in the Medial Prefrontal Cortex.Front Psychiatry. 2020 Sep 3;11:837. doi: 10.3389/fpsyt.2020.00837. eCollection 2020. Front Psychiatry. 2020. PMID: 33088274 Free PMC article.
-
Mapping the central effects of ketamine in the rat using pharmacological MRI.Psychopharmacology (Berl). 2006 May;186(1):64-81. doi: 10.1007/s00213-006-0344-0. Epub 2006 Mar 21. Psychopharmacology (Berl). 2006. PMID: 16550385
-
Intrinsic Connectivity Networks of Glutamate-Mediated Antidepressant Response: A Neuroimaging Review.Front Psychiatry. 2022 May 26;13:864902. doi: 10.3389/fpsyt.2022.864902. eCollection 2022. Front Psychiatry. 2022. PMID: 35722550 Free PMC article. Review.
References
-
- Abi-Saab WM, D'Souza DC, Moghaddam B, Krystal JH (1998) The NMDA antagonist model for schizophrenia: promise and pitfalls. Pharmacopsychiatry 31 [Suppl 2]: 104-109. - PubMed
-
- Adler CM, Malhotra AK, Elman I, Goldberg T, Egan M, Pickar D, Breier A (1999) Comparison of ketamine-induced thought disorder in healthy volunteers and thought disorder in schizophrenia. Am J Psychiatry 156: 1646-1649. - PubMed
-
- Al-Amin HA, Shannon Weickert C, Weinberger DR, Lipska BK (2001) Delayed onset of enhanced MK-801-induced motor hyperactivity after neonatal lesions of the rat ventral hippocampus. Biol Psychiatry 49: 528-539. - PubMed
-
- Anwyl R, Mulkeen D, Rowan MJ (1989) The role of N-methyl-d-aspartate receptors in the generation of short-term potentiation in the rat hippocampus. Brain Res 503: 148-151. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
