Differential role of inhibition in habituation of two independent afferent pathways to a common motor output
- PMID: 15647595
- PMCID: PMC548496
- DOI: 10.1101/lm.83405
Differential role of inhibition in habituation of two independent afferent pathways to a common motor output
Abstract
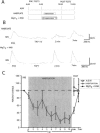
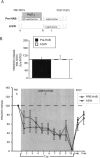
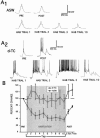
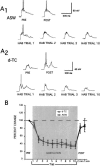
Many studies of the neural mechanisms of learning have focused on habituation, a simple form of learning in which a response decrements with repeated stimulation. In the siphon-elicited siphon withdrawal reflex (S-SWR) of the marine mollusk Aplysia, the prevailing view is that homosynaptic depression of primary sensory afferents underlies short-term habituation. Here we examined whether this mechanism is also utilized in habituation of the tail-elicited siphon withdrawal reflex (T-SWR), which is triggered by an independent, polysynaptic afferent pathway that converges onto the same siphon motor neurons (MNs). By using semi-intact preparations in which tail and/or siphon input to siphon MNs could be measured, we found that repeated tail stimuli administered in the presence of a reversible conduction block of the nerves downstream of the tail sensory neurons (SNs) completely abolished the induction of habituation. Subsequent retraining revealed no evidence of savings, indicating that the tail SNs and their immediate interneuronal targets are not the locus of plasticity underlying T-SWR habituation. The networks closely associated with the siphon MNs are modulated by cholinergic inhibition. We next examined the effects of network disinhibition on S-SWR and T-SWR habituation using an Ach receptor antagonist d-tubocurarine. We found that the resulting network disinhibition disrupted T-SWR, but not S-SWR, habituation. Indeed, repeated tail stimulation in the presence of d-tubocurarine resulted in an initial enhancement in responding. Lastly, we tested whether habituation of T-SWR generalized to S-SWR and found that it did not. Collectively, these data indicate that (1) unlike S-SWR, habituation of T-SWR does not involve homosynaptic depression of SNs; and (2) the sensitivity of T-SWR habituation to network disinhibition is consistent with an interneuronal plasticity mechanism that is unique to the T-SWR circuit, since it does not alter S-SWR.
Figures


References
-
- Abramson, C.I. 1994. A primer of invertebrate learning: A behavioral perspective. American Psychological Association, Washington, DC.
-
- Bailey, C.H. and Chen, M. 1983. Morphological basis of long-term habituation and sensitization in Aplysia. Science 220: 91-93. - PubMed
-
- ———. 1989. Structural plasticity at identified synapses during long-term memory in Aplysia. J. Neurobiol. 20: 356-372. - PubMed
-
- Belkin, K.J. and Abrams, T.W. 1998. The effect of the neuropeptide FMRFamide on Aplysia californica siphon motoneurons involves multiple ionic currents that vary seasonally. J. Exp. Biol. 201: 2225-2234. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
