Subunit-specific gating controls rat NR1/NR2A and NR1/NR2B NMDA channel kinetics and synaptic signalling profiles
- PMID: 15649985
- PMCID: PMC1665591
- DOI: 10.1113/jphysiol.2004.080028
Subunit-specific gating controls rat NR1/NR2A and NR1/NR2B NMDA channel kinetics and synaptic signalling profiles
Abstract
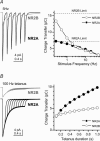
NR2A and NR2B are the predominant NR2 NMDA receptor subunits expressed in cortex and hippocampus. The relative expression level of NR2A and NR2B is regulated developmentally and these two subunits have been suggested to play distinct roles in long-term synaptic plasticity. We have used patch-clamp recording of recombinant NMDA receptors expressed in HEK293 cells to characterize the activation properties of both NR1/NR2A and NR1/NR2B receptors. Recordings from outside-out patches that contain a single active channel show that NR2A-containing receptors have a higher probability of opening at least once in response to a brief synaptic-like pulse of glutamate than NR2B-containing receptors (NR2A, 0.80; NR2B, 0.56), a higher peak open probability (NR2A, 0.50; NR2B, 0.12), and a higher open probability within an activation (NR2A, 0.67; NR2B, 0.37). Analysis of the sequence of single-channel open and closed intervals shows that both NR2A- and NR2B-containing receptors undergo multiple conformational changes prior to opening of the channel, with at least one of these steps being faster for NR2A than NR2B. These distinct properties produce profoundly different temporal signalling profiles for NR2A- and NR2B-containing receptors. Simulations of synaptic responses demonstrate that at low frequencies typically used to induce long-term depression (LTD; 1 Hz), NR1/NR2B makes a larger contribution to total charge transfer and therefore calcium influx than NR1/NR2A. However, under high-frequency tetanic stimulation (100 Hz; > 100 ms) typically used to induce long-term potentiation (LTP), the charge transfer mediated by NR1/NR2A considerably exceeds that of NR1/NR2B.
Figures



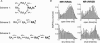



References
-
- Armstrong N, Gouaux E. Mechanisms for activation and antagonism of an AMPA-sensitive glutamate receptor: crystal structures of the GluR2 ligand binding core. Neuron. 2000;28:165–181. 10.1016/S0896-6273(00)00094-5. - DOI - PubMed
-
- Banke TG, Traynelis SF. Activation of NR1/NR2B NMDA receptors. Nat Neurosci. 2003;6:144–152. - PubMed
-
- Brauner-Osborne H, Egebjerg J, Nielsen EO, Madsen U, Krogsgaard-Larsen P. Ligands for glutamate receptors: design and therapeutic prospects. J Med Chem. 2000;43:2609–2645. - PubMed
-
- Carmignoto G, Vicini S. Activity-dependent decrease in NMDA receptor responses during development of the visual cortex. Science. 1992;258:1007–1011. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

