AML1-ETO and C-KIT mutation/overexpression in t(8;21) leukemia: implication in stepwise leukemogenesis and response to Gleevec
- PMID: 15650049
- PMCID: PMC545849
- DOI: 10.1073/pnas.0408831102
AML1-ETO and C-KIT mutation/overexpression in t(8;21) leukemia: implication in stepwise leukemogenesis and response to Gleevec
Abstract
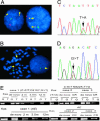
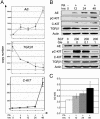
To explore the genetic abnormalities that cooperate with AML1-ETO (AE) fusion gene to cause acute myeloid leukemia (AML) with t(8;21), we screened a number of candidate genes and identified 11 types of mutations in C-KIT gene (mC-KIT), including 6 previously undescribed ones among 26 of 54 (48.1%) cases with t(8;21). To address a possible chronological order between AE and mC-KIT, we showed that, among patients with AE and mC-KIT, most leukemic cells at disease presentation harbored both genetic alteration, whereas in three such cases investigated during complete remission, only AE, but not mC-KIT, could be detected by allele-specific PCR. Therefore, mC-KIT should be a subsequent event on the basis of t(8;21). Furthermore, induced expression of AE in U937-A/E cells significantly up-regulated mRNA and protein levels of C-KIT. This may lead to an alternative way of C-KIT activation and may explain the significantly higher C-KIT expression in 81.3% of patients with t(8;21) than in patients with other leukemias. These data strongly suggest that t(8;21) AML follows a stepwise model in leukemogenesis, i.e., AE represents the first, fundamental genetic hit to initiate the disease, whereas activation of the C-KIT pathway may be a second but also crucial hit for the development of a full-blown leukemia. Additionally, Gleevec suppressed the C-KIT activity and induced proliferation inhibition and apoptosis in cells bearing C-KIT N822K mutation or overexpression, but not in cells with D816 mC-KIT. Gleevec also exerted a synergic effect in apoptosis induction with cytarabine, thus providing a potential therapeutic for t(8;21) leukemia.
Figures


References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

