Herpes simplex virus 1 infection activates the endoplasmic reticulum resident kinase PERK and mediates eIF-2alpha dephosphorylation by the gamma(1)34.5 protein
- PMID: 15650164
- PMCID: PMC544103
- DOI: 10.1128/JVI.79.3.1379-1388.2005
Herpes simplex virus 1 infection activates the endoplasmic reticulum resident kinase PERK and mediates eIF-2alpha dephosphorylation by the gamma(1)34.5 protein
Abstract
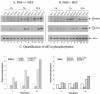
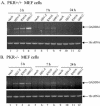
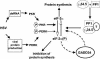
The gamma(1)34.5 protein of herpes simplex virus (HSV) plays a crucial role in virus infection. Although the double-stranded RNA-dependent protein kinase (PKR) is activated during HSV infection, the gamma(1)34.5 protein inhibits the activity of PKR by mediating dephosphorylation of the translation initiation factor eIF-2alpha. Here we show that HSV infection also induces phosphorylation of an endoplasmic reticulum (ER) resident kinase PERK, a hallmark of ER stress response. The virus-induced phosphorylation of PERK is blocked by cycloheximide but not by phosphonoacetic acid, suggesting that the accumulation of viral proteins in the ER is essential. Notably, the maximal phosphorylation of PERK is delayed in PKR+/+ cells compared to that seen in PKR-/- cells. Further analysis indicates that hyperphosphorylation of eIF-2alpha caused by HSV is greater in PKR+/+ cells than in PKR-/- cells. However, expression of the gamma(1)34.5 protein suppresses the ER stress response caused by virus, dithiothreitol, and thapsigargin as measured by global protein synthesis. Interestingly, the expression of GADD34 stimulated by HSV infection parallels the status of eIF-2alpha phosphorylation. Together, these observations suggest that regulation of eIF-2alpha phosphorylation by the gamma(1)34.5 protein is an efficient way to antagonize the inhibitory activity of PKR as well as PERK during productive infection.
Figures


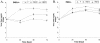
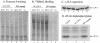
Similar articles
-
The herpes simplex virus US11 protein effectively compensates for the gamma1(34.5) gene if present before activation of protein kinase R by precluding its phosphorylation and that of the alpha subunit of eukaryotic translation initiation factor 2.J Virol. 1998 Nov;72(11):8620-6. doi: 10.1128/JVI.72.11.8620-8626.1998. J Virol. 1998. PMID: 9765401 Free PMC article.
-
The gamma(1)34.5 protein of herpes simplex virus 1 complexes with protein phosphatase 1alpha to dephosphorylate the alpha subunit of the eukaryotic translation initiation factor 2 and preclude the shutoff of protein synthesis by double-stranded RNA-activated protein kinase.Proc Natl Acad Sci U S A. 1997 Feb 4;94(3):843-8. doi: 10.1073/pnas.94.3.843. Proc Natl Acad Sci U S A. 1997. PMID: 9023344 Free PMC article.
-
Resistance of mRNA translation to acute endoplasmic reticulum stress-inducing agents in herpes simplex virus type 1-infected cells requires multiple virus-encoded functions.J Virol. 2006 Aug;80(15):7354-63. doi: 10.1128/JVI.00479-06. J Virol. 2006. PMID: 16840316 Free PMC article.
-
Viral proteins targeting host protein kinase R to evade an innate immune response: a mini review.Biotechnol Genet Eng Rev. 2018 Apr;34(1):33-59. doi: 10.1080/02648725.2018.1467151. Epub 2018 May 2. Biotechnol Genet Eng Rev. 2018. PMID: 29716441 Review.
-
Small molecule modulators of eukaryotic initiation factor 2α kinases, the key regulators of protein synthesis.Biochimie. 2013 Nov;95(11):1980-90. doi: 10.1016/j.biochi.2013.07.030. Epub 2013 Aug 11. Biochimie. 2013. PMID: 23939221 Review.
Cited by
-
Arms Race between Enveloped Viruses and the Host ERAD Machinery.Viruses. 2016 Sep 19;8(9):255. doi: 10.3390/v8090255. Viruses. 2016. PMID: 27657106 Free PMC article. Review.
-
Differential unfolded protein response during Chikungunya and Sindbis virus infection: CHIKV nsP4 suppresses eIF2α phosphorylation.Virol J. 2013 Jan 28;10:36. doi: 10.1186/1743-422X-10-36. Virol J. 2013. PMID: 23356742 Free PMC article.
-
Activation of the unfolded protein response by 2-deoxy-D-glucose inhibits Kaposi's sarcoma-associated herpesvirus replication and gene expression.Antimicrob Agents Chemother. 2012 Nov;56(11):5794-803. doi: 10.1128/AAC.01126-12. Epub 2012 Aug 27. Antimicrob Agents Chemother. 2012. PMID: 22926574 Free PMC article.
-
KSHV activates unfolded protein response sensors but suppresses downstream transcriptional responses to support lytic replication.PLoS Pathog. 2019 Dec 2;15(12):e1008185. doi: 10.1371/journal.ppat.1008185. eCollection 2019 Dec. PLoS Pathog. 2019. PMID: 31790507 Free PMC article.
-
Regulation of host translational machinery by African swine fever virus.PLoS Pathog. 2009 Aug;5(8):e1000562. doi: 10.1371/journal.ppat.1000562. Epub 2009 Aug 28. PLoS Pathog. 2009. PMID: 19714237 Free PMC article.
References
-
- Bertolotti, A., Y. Zhang, L. M. Hendershot, H. P. Harding, and D. Ron. 2000. Dynamic interaction of BiP and ER stress transducers in the unfolded-protein response. Nat. Cell Biol. 2:326-332. - PubMed
-
- Cerveny, M., S. Hessefort, K. Yang, G. Cheng, M. Gross, and B. He. 2003. Amino acid substitutions in the effector domain of the γ134.5 protein of herpes simplex virus 1 have differential effects on viral response to interferon-α. Virology 307:290-300. - PubMed
-
- Cheng, G., M. E. Brett, and B. He. 2001. Val193 and Phe195 of the γ134.5 protein of herpes simplex virus 1 are required for viral resistance to interferon α/β. Virology 290:115-120. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

