Mutual interference between genomic RNA replication and subgenomic mRNA transcription in brome mosaic virus
- PMID: 15650170
- PMCID: PMC544081
- DOI: 10.1128/JVI.79.3.1438-1451.2005
Mutual interference between genomic RNA replication and subgenomic mRNA transcription in brome mosaic virus
Abstract
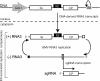
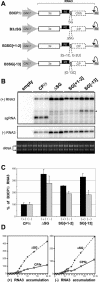
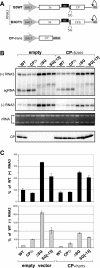
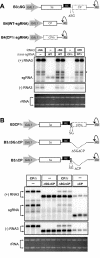
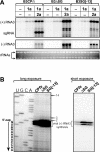
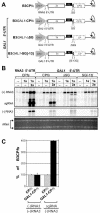
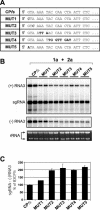
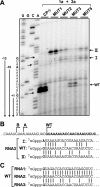
Replication by many positive-strand RNA viruses includes genomic RNA amplification and subgenomic mRNA (sgRNA) transcription. For brome mosaic virus (BMV), both processes occur in virus-induced, membrane-associated compartments, require BMV replication factors 1a and 2a, and use negative-strand RNA3 as a template for genomic RNA3 and sgRNA syntheses. To begin elucidating their relations, we examined the interaction of RNA3 replication and sgRNA transcription in Saccharomyces cerevisiae expressing 1a and 2a, which support the full RNA3 replication cycle. Blocking sgRNA transcription stimulated RNA3 replication by up to 350%, implying that sgRNA transcription inhibits RNA3 replication. Such inhibition was independent of the sgRNA-encoded coat protein and operated in cis. We further found that sgRNA transcription inhibited RNA3 replication at a step or steps after negative-strand RNA3 synthesis, implying competition with positive-strand RNA3 synthesis for negative-strand RNA3 templates, viral replication factors, or common host components. Consistent with this, sgRNA transcription was stimulated by up to 400% when mutations inhibiting positive-strand RNA3 synthesis were introduced into the RNA3 5'-untranslated region. Thus, BMV subgenomic and genomic RNA syntheses mutually interfered with each other, apparently by competition for one or more common factors. In plant protoplasts replicating all three BMV genomic RNAs, mutations blocking sgRNA transcription often had lesser effects on RNA3 accumulation, possibly because RNA3 also competed with RNA1 and RNA2 replication templates and because any increase in RNA3 replication at the expense of RNA1 and RNA2 would be self-limited by decreased 1a and 2a expression from RNA1 and RNA2.
Figures

Similar articles
-
Replicase-binding sites on plus- and minus-strand brome mosaic virus RNAs and their roles in RNA replication in plant cells.J Virol. 2004 Dec;78(24):13420-9. doi: 10.1128/JVI.78.24.13420-13429.2004. J Virol. 2004. PMID: 15564452 Free PMC article.
-
In vivo DNA expression of functional brome mosaic virus RNA replicons in Saccharomyces cerevisiae.J Virol. 1997 Oct;71(10):7781-90. doi: 10.1128/JVI.71.10.7781-7790.1997. J Virol. 1997. PMID: 9311863 Free PMC article.
-
Brome mosaic virus Protein 1a recruits viral RNA2 to RNA replication through a 5' proximal RNA2 signal.J Virol. 2001 Apr;75(7):3207-19. doi: 10.1128/JVI.75.7.3207-3219.2001. J Virol. 2001. PMID: 11238847 Free PMC article.
-
The coat protein leads the way: an update on basic and applied studies with the Brome mosaic virus coat protein.Mol Plant Pathol. 2011 May;12(4):403-12. doi: 10.1111/j.1364-3703.2010.00678.x. Epub 2010 Nov 25. Mol Plant Pathol. 2011. PMID: 21453435 Free PMC article. Review.
-
Common replication strategies emerging from the study of diverse groups of positive-strand RNA viruses.Arch Virol Suppl. 1994;9:181-94. doi: 10.1007/978-3-7091-9326-6_18. Arch Virol Suppl. 1994. PMID: 8032249 Review.
Cited by
-
Nuclear Protein Sam68 Interacts with the Enterovirus 71 Internal Ribosome Entry Site and Positively Regulates Viral Protein Translation.J Virol. 2015 Oct;89(19):10031-43. doi: 10.1128/JVI.01677-15. Epub 2015 Jul 22. J Virol. 2015. PMID: 26202240 Free PMC article.
-
Systematic identification of novel, essential host genes affecting bromovirus RNA replication.PLoS One. 2011;6(8):e23988. doi: 10.1371/journal.pone.0023988. Epub 2011 Aug 22. PLoS One. 2011. PMID: 21915247 Free PMC article.
-
Distinct sites on the Sindbis virus RNA-dependent RNA polymerase for binding to the promoters for the synthesis of genomic and subgenomic RNA.J Virol. 2007 Apr;81(8):4371-3. doi: 10.1128/JVI.02672-06. Epub 2007 Feb 7. J Virol. 2007. PMID: 17287268 Free PMC article.
-
Organelle luminal dependence of (+)strand RNA virus replication reveals a hidden druggable target.Sci Adv. 2018 Jan 24;4(1):eaap8258. doi: 10.1126/sciadv.aap8258. eCollection 2018 Jan. Sci Adv. 2018. PMID: 29387794 Free PMC article.
-
Characterization of a novel 5' subgenomic RNA3a derived from RNA3 of Brome mosaic bromovirus.J Virol. 2006 Dec;80(24):12357-66. doi: 10.1128/JVI.01207-06. Epub 2006 Sep 27. J Virol. 2006. PMID: 17005659 Free PMC article.
References
-
- Adkins, S., and C. C. Kao. 1998. Subgenomic RNA promoters dictate the mode of recognition by bromoviral RNA-dependent RNA polymerases. Virology 252:1-8. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

