Vaccination of rhesus macaques with recombinant Mycobacterium bovis bacillus Calmette-Guérin Env V3 elicits neutralizing antibody-mediated protection against simian-human immunodeficiency virus with a homologous but not a heterologous V3 motif
- PMID: 15650171
- PMCID: PMC544111
- DOI: 10.1128/JVI.79.3.1452-1462.2005
Vaccination of rhesus macaques with recombinant Mycobacterium bovis bacillus Calmette-Guérin Env V3 elicits neutralizing antibody-mediated protection against simian-human immunodeficiency virus with a homologous but not a heterologous V3 motif
Abstract
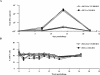
Although the correlates of vaccine-induced protection against human immunodeficiency virus type 1 (HIV-1) are not fully known, it is presumed that neutralizing antibodies (NAb) play a role in controlling virus infection. In this study, we examined immune responses elicited in rhesus macaques following vaccination with recombinant Mycobacterium bovis bacillus Calmette-Guérin expressing an HIV-1 Env V3 antigen (rBCG Env V3). We also determined the effect of vaccination on protection against challenge with either a simian-human immunodeficiency virus (SHIV-MN) or a highly pathogenic SHIV strain (SHIV-89.6PD). Immunization with rBCG Env V3 elicited significant levels of NAb for the 24 weeks tested that were predominantly HIV-1 type specific. Sera from the immunized macaques neutralized primary HIV-1 isolates in vitro, including HIV-1BZ167/X4, HIV-1SF2/X4, HIV-1CI2/X4, and, to a lesser extent, HIV-1MNp/X4, all of which contain a V3 sequence homologous to that of rBCG Env V3. In contrast, neutralization was not observed against HIV-1SF33/X4, which has a heterologous V3 sequence, nor was it found against primary HIV-1 R5 isolates from either clade A or B. Furthermore, the viral load in the vaccinated macaques was significantly reduced following low-dose challenge with SHIV-MN, and early plasma viremia was markedly decreased after high-dose SHIV-MN challenge. In contrast, replication of pathogenic SHIV-89.6PD was not affected by vaccination in any of the macaques. Thus, we have shown that immunization with an rBCG Env V3 vaccine elicits a strong, type-specific V3 NAb response in rhesus macaques. While this response was not sufficient to provide protection against a pathogenic SHIV challenge, it was able to significantly reduce the viral load in macaques following challenge with a nonpathogenic SHIV. These observations suggest that rBCG vectors have the potential to deliver an appropriate virus immunogen for desirable immune elicitations.
Figures

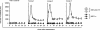




References
-
- Aldovini, A., and R. A. Young. 1991. Humoral and cell-mediated immune responses to live recombinant BCG-HIV vaccines. Nature 351:479-482. - PubMed
-
- Amara, R. R., F. Villinger, J. D. Altman, S. L. Lydy, S. P. O'Neil, S. I. Staprans, D. C. Montefiori, Y. Xu, J. G. Herndon, L. S. Wyatt, M. A. Candido, N. L. Kozyr, P. L. Earl, J. M. Smith, H. L. Ma, B. D. Grimm, M. L. Hulsey, J. Miller, H. M. McClure, J. M. McNicholl, B. Moss, and H. L. Robinson. 2001. Control of a mucosal challenge and prevention of AIDS by a multiprotein DNA/MVA vaccine. Science 292:69-74. - PubMed
-
- Baba, T. W., V. Liska, A. H. Khimani, N. B. Ray, P. J. Dailey, D. Penninck, R. Bronson, M. F. Greene, H. M. McClure, L. N. Martin, and R. M. Ruprecht. 1999. Live attenuated, multiply deleted simian immunodeficiency virus causes AIDS in infant and adult macaques. Nat. Med. 5:194-203. - PubMed
-
- Baba, T. W., Y. S. Jeong, D. Pennick, R. Bronson, M. F. Greene, and R. M. Ruprecht. 1995. Pathogenicity of live, attenuated SIV after mucosal infection of neonatal macaques. Science 267:1820-1825. - PubMed
-
- Barouch, D. H., S. Santra, J. E. Schmitz, M. J. Kuroda, T. M. Fu, W. Wagner, M. Bilska, A. Craiu, X. X. Zheng, G. R. Krivulka, K. Beaudry, M. A. Lifton, C. E. Nickerson, W. L. Trigona, K. Punt, D. C. Freed, L. Guan, S. Dubey, D. Casimiro, A. Simon, M. E. Davies, M. Chastain, T. B. Strom, R. S. Gelman, D. C. Montefiori, M. G. Lewis, E. A. Emini, J. W. Shiver, and N. L. Letvin. 2000. Control of viremia and prevention of clinical AIDS in rhesus monkeys by cytokine-augmented DNA vaccination. Science 290:486-492. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

