CCR5, GPR15, and CXCR6 are major coreceptors of human immunodeficiency virus type 2 variants isolated from individuals with and without plasma viremia
- PMID: 15650194
- PMCID: PMC544080
- DOI: 10.1128/JVI.79.3.1686-1700.2005
CCR5, GPR15, and CXCR6 are major coreceptors of human immunodeficiency virus type 2 variants isolated from individuals with and without plasma viremia
Abstract
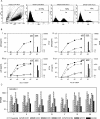
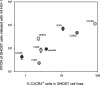
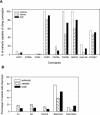
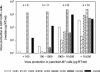
Human immunodeficiency virus type 2 (HIV-2) is generally considered capable of using a broad range of coreceptors. Since HIV-2 variants from individuals with nonprogressive infection were not studied previously, the possibility that broad coreceptor usage is a property of variants associated with progressive infection could not be excluded. To test this, we determined the coreceptor usage of 43 HIV-2 variants isolated from six long-term-infected individuals with undetectable plasma viremia. Using GHOST indicator cells, we showed for the first time that the only coreceptors efficiently used by low-pathogenic HIV-2 variants are CCR5, GPR15 (BOB), and CXCR6 (BONZO). Surprisingly, control HIV-2 variants (n = 45) isolated from seven viremic individuals also mainly used these three coreceptors, whereas use of CCR1, CCR2b, or CCR3 was rare. Nearly a quarter of all HIV-2 variants tested could infect the parental GHOST cells, which could be partially explained by CXCR4 usage. Use of CXCR4 was observed only for HIV-2 variants from viremic individuals. Thirty-eight variants from aviremic and viremic HIV-2-infected individuals were additionally tested in U87 cells. All except one were capable of infecting the parental U87 cells, often with high efficiency. When virus production in parental cells was regarded as background in the coreceptor-transduced cell lines, the results in U87 cells were largely in agreement with the findings in GHOST cells. HIV-2 isolates from aviremic individuals commonly use as coreceptors CCR5, GPR15, and CXCR6, as well as an unidentified receptor expressed by U87 cells. Broad coreceptor usage, therefore, does not appear to be associated with pathogenicity of HIV-2.
Figures


References
-
- Albert, J., A. Naucler, B. Bottiger, P. A. Broliden, P. Albino, S. A. Ouattara, C. Bjorkegren, A. Valentin, G. Biberfeld, and E. M. Fenyo. 1990. Replicative capacity of HIV-2, like HIV-1, correlates with severity of immunodeficiency. AIDS 4:291-295. - PubMed
-
- Berkowitz, R. D., S. Alexander, C. Bare, V. Linquist-Stepps, M. Bogan, M. E. Moreno, L. Gibson, E. D. Wieder, J. Kosek, C. A. Stoddart, and J. M. McCune. 1998. CCR5- and CXCR4-utilizing strains of human immunodeficiency virus type 1 exhibit differential tropism and pathogenesis in vivo. J. Virol. 72:10108-10117. - PMC - PubMed
-
- Berry, N., K. Ariyoshi, S. Jaffar, S. Sabally, T. Corrah, R. Tedder, and H. Whittle. 1998. Low peripheral blood viral HIV-2 RNA in individuals with high CD4 percentage differentiates HIV-2 from HIV-1 infection. J. Hum. Virol. 1:457-468. - PubMed
-
- Blaak, H., P. H. Boers, M. Schutten, M. E. Van Der Ende, and A. D. Osterhaus. 2004. HIV-2-infected individuals with undetectable plasma viremia carry replication-competent virus in peripheral blood lymphocytes. J. Acquir. Immun. Defic. Syndr. 36:777-782. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

