Glc1.8 from Microplitis demolitor bracovirus induces a loss of adhesion and phagocytosis in insect high five and S2 cells
- PMID: 15650210
- PMCID: PMC544146
- DOI: 10.1128/JVI.79.3.1861-1870.2005
Glc1.8 from Microplitis demolitor bracovirus induces a loss of adhesion and phagocytosis in insect high five and S2 cells
Abstract
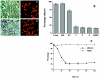
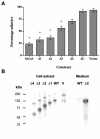
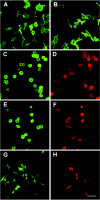
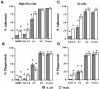
Polydnaviridae is a unique family of DNA viruses that are symbiotically associated with parasitoid wasps. Upon oviposition, wasps inject these viruses into their hosts, where they cause several physiological alterations, including suppression of the cellular immune response. Here we report that expression of the glc1.8 gene from Microplitis demolitor bracovirus (MdBV) causes a loss of adhesion by two hemocyte-like cell lines, namely, High Five cells from the lepidopteran Trichoplusia ni and S2 cells from the dipteran Drosophila melanogaster. The expression of recombinant Glc1.8 also greatly reduced the ability of these cells to phagocytize foreign targets. Glc1.8 is characterized by a signal peptide at its N terminus, an extracellular domain comprised of five nearly perfect tandem repeats of 78 amino acids, and a C-terminal hydrophobic domain that encodes a putative membrane anchor sequence. The expression of a Glc1.8 mutant lacking the anchor sequence resulted in a secreted protein that had no effect on adhesion or phagocytosis. In contrast, sequential deletion of the repeats in the extracellular domain resulted in a progressive reduction in immunosuppressive activity. Since each repeat and its associated glycosylation sites are nearly identical, these results suggested that adhesion-blocking activity depends more on the overall number of repeats in the extracellular domain than on the specific determinants within each repeat. While it severely compromised adhesion and phagocytic functions, Glc1.8 did not cause cell death. Collectively, these results indicate that Glc1.8 is a major pathogenic determinant of MdBV that is involved in suppression of the insect cellular immune response.
Figures



References
-
- Asgari, S., M. Hellers, and O. Schmidt. 1996. Host haemocyte inactivation by an insect parasitoid: transient expression of a polydnavirus gene. J. Gen. Virol. 77:2653-2662. - PubMed
-
- Asgari, S., O. Schmidt, and U. Theopold. 1997. A polydnavirus-encoded protein of an endoparasitoid wasp is an immune suppressor. J. Gen. Virol. 78:3061-3070. - PubMed
-
- Beck, M., and M. R. Strand. 2003. RNA interference silences Microplitis demolitor bracovirus genes and implicates glc1.8 in blocking adhesion of infected host cells. Virology 314:521-535. - PubMed
-
- Blander, J. M., and R. Medzhitov. 2004. Regulation of phagosome maturation by signals from Toll-like receptors. Science 304:1014-1018. - PubMed
-
- Chervenak, J. L., and N. P. Illsley. 2000. Episialin acts as an antiadhesive factor in an in vitro model of human endometrial-blastocyst attachment. Biol. Reprod. 63:294-300. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

