Exposure of RNA templates and encapsidation of spliced viral RNA are influenced by the arginine-rich domain of human hepatitis B virus core antigen (HBcAg 165-173)
- PMID: 15650211
- PMCID: PMC544126
- DOI: 10.1128/JVI.79.3.1871-1887.2005
Exposure of RNA templates and encapsidation of spliced viral RNA are influenced by the arginine-rich domain of human hepatitis B virus core antigen (HBcAg 165-173)
Abstract
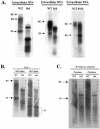
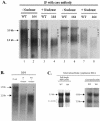
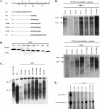
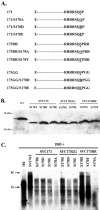
Previously, human hepatitis B virus (HBV) mutant 164, which has a truncation at the C terminus of the HBV core antigen (HBcAg), was speculated to secrete immature genomes. For this study, we further characterized mutant 164 by different approaches. In addition to the 3.5-kb pregenomic RNA (pgRNA), the mutant preferentially encapsidated the 2.2-kb or shorter species of spliced RNA, which can be reverse transcribed into double-stranded DNA before virion secretion. We observed that mutant 164 produced less 2.2-kb spliced RNA than the wild type. Furthermore, it appeared to produce at least two different populations of capsids: one encapsidated a nuclease-sensitive 3.5-kb pgRNA while the other encapsidated a nuclease-resistant 2.2-kb spliced RNA. In contrast, the wild-type core-associated RNA appeared to be resistant to nuclease. When arginines and serines were systematically restored at the truncated C terminus, the core-associated DNA and nuclease-resistant RNA gradually increased in both size and signal intensity. Full protection of encapsidated pgRNA from nuclease was observed for HBcAg 1-171. A full-length positive-strand DNA phenotype requires positive charges at amino acids 172 and 173. Phosphorylation at serine 170 is required for optimal RNA encapsidation and a full-length positive-strand DNA phenotype. RNAs encapsidated in Escherichia coli by capsids of HBcAg 154, 164, and 167, but not HBcAg 183, exhibited nuclease sensitivity; however, capsid instability after nuclease treatment was observed only for HBcAg 164 and 167. A new hypothesis is proposed here to highlight the importance of a balanced charge density for capsid stability and intracapsid anchoring of RNA templates.
Figures





Similar articles
-
Carboxy-terminal truncations of the HBV core protein affect capsid formation and the apparent size of encapsidated HBV RNA.Virology. 1993 Jun;194(2):597-607. doi: 10.1006/viro.1993.1299. Virology. 1993. PMID: 7684872
-
Hepatitis B virus nucleocapsids formed by carboxy-terminally mutated core proteins contain spliced viral genomes but lack full-size DNA.J Virol. 2004 Dec;78(24):13812-8. doi: 10.1128/JVI.78.24.13812-13818.2004. J Virol. 2004. PMID: 15564489 Free PMC article.
-
Testing an electrostatic interaction hypothesis of hepatitis B virus capsid stability by using an in vitro capsid disassembly/reassembly system.J Virol. 2009 Oct;83(20):10616-26. doi: 10.1128/JVI.00749-09. Epub 2009 Aug 5. J Virol. 2009. PMID: 19656897 Free PMC article.
-
Phosphorylation of the Arginine-Rich C-Terminal Domains of the Hepatitis B Virus (HBV) Core Protein as a Fine Regulator of the Interaction between HBc and Nucleic Acid.Viruses. 2020 Jul 8;12(7):738. doi: 10.3390/v12070738. Viruses. 2020. PMID: 32650547 Free PMC article. Review.
-
Structure and function of the encapsidation signal of hepadnaviridae.J Viral Hepat. 1998 Nov;5(6):357-67. doi: 10.1046/j.1365-2893.1998.00124.x. J Viral Hepat. 1998. PMID: 9857345 Review.
Cited by
-
Naturally occurring core immune-escape and carboxy-terminal mutations\truncations in patients with e antigen negative chronic hepatitis B.Hepatol Int. 2012 Oct;6(4):707-17. doi: 10.1007/s12072-011-9316-5. Epub 2011 Dec 2. Hepatol Int. 2012. PMID: 26201521
-
Hepatitis B virus nucleocapsid but not free core antigen controls viral clearance in mice.J Virol. 2012 Sep;86(17):9266-73. doi: 10.1128/JVI.00608-12. Epub 2012 Jun 20. J Virol. 2012. PMID: 22718814 Free PMC article.
-
Preparation by alkaline treatment and detailed characterisation of empty hepatitis B virus core particles for vaccine and gene therapy applications.Sci Rep. 2015 Jun 26;5:11639. doi: 10.1038/srep11639. Sci Rep. 2015. PMID: 26113394 Free PMC article.
-
Testing the balanced electrostatic interaction hypothesis of hepatitis B virus DNA synthesis by using an in vivo charge rebalance approach.J Virol. 2010 Mar;84(5):2340-51. doi: 10.1128/JVI.01666-09. Epub 2009 Dec 16. J Virol. 2010. PMID: 20015989 Free PMC article.
-
Hepatitis B virus core antigen determines viral persistence in a C57BL/6 mouse model.Proc Natl Acad Sci U S A. 2010 May 18;107(20):9340-5. doi: 10.1073/pnas.1004762107. Epub 2010 May 3. Proc Natl Acad Sci U S A. 2010. PMID: 20439715 Free PMC article.
References
-
- Beames, B., and R. E. Lanford. 1993. Carboxy-terminal truncations of the HBV core protein affect capsid formation and the apparent size of encapsidated HBV RNA. Virology 194:597-607. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

