Enhanced cytotoxicity without internuclear spread of adenovirus upon cell fusion by measles virus glycoproteins
- PMID: 15650215
- PMCID: PMC544120
- DOI: 10.1128/JVI.79.3.1911-1917.2005
Enhanced cytotoxicity without internuclear spread of adenovirus upon cell fusion by measles virus glycoproteins
Abstract
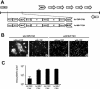
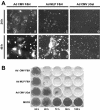
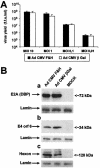
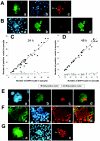
The efficiency of viruses in cancer therapy is enhanced by proteins that mediate the fusion of infected cells with their neighbors. It was reported that replication-competent adenovirus particles can spread between nuclei within fusion-generated syncytia. To assess this conjecture, we generated fusogenic adenoviruses that express a balanced ratio of the F and H glycoproteins of measles virus. The viruses displayed enhanced cytotoxicity but largely unchanged replication efficiencies compared to a nonfusogenic virus. Most notably, the virus genomes did not spread through fusion-generated multinuclear cells. Hence, adenovirus replication in syncytia remains largely restricted to initially transduced nuclei.
Figures

Similar articles
-
Importance of the cytoplasmic tails of the measles virus glycoproteins for fusogenic activity and the generation of recombinant measles viruses.J Virol. 2002 Jul;76(14):7174-86. doi: 10.1128/jvi.76.14.7174-7186.2002. J Virol. 2002. PMID: 12072517 Free PMC article.
-
Measles virus: both the haemagglutinin and fusion glycoproteins are required for fusion.J Gen Virol. 1991 Feb;72 ( Pt 2):439-42. doi: 10.1099/0022-1317-72-2-439. J Gen Virol. 1991. PMID: 1993882
-
Use of viral fusogenic membrane glycoproteins as novel therapeutic transgenes in gliomas.Hum Gene Ther. 2001 May 1;12(7):811-21. doi: 10.1089/104303401750148766. Hum Gene Ther. 2001. PMID: 11339897
-
Virus-Mediated Cell-Cell Fusion.Int J Mol Sci. 2020 Dec 17;21(24):9644. doi: 10.3390/ijms21249644. Int J Mol Sci. 2020. PMID: 33348900 Free PMC article. Review.
-
Nuclear organization of replication and gene expression in adenovirus-infected cells.Curr Top Microbiol Immunol. 1995;199 ( Pt 1):99-117. doi: 10.1007/978-3-642-79496-4_7. Curr Top Microbiol Immunol. 1995. PMID: 7555063 Review. No abstract available.
Cited by
-
Use of cell fusion proteins to enhance adenoviral vector efficacy as an anti-cancer therapeutic.Cancer Gene Ther. 2021 Aug;28(7-8):745-756. doi: 10.1038/s41417-020-0192-9. Epub 2020 Jul 1. Cancer Gene Ther. 2021. PMID: 32606392 Review.
-
Principles of Virus Uncoating: Cues and the Snooker Ball.Traffic. 2016 Jun;17(6):569-92. doi: 10.1111/tra.12387. Epub 2016 Mar 31. Traffic. 2016. PMID: 26875443 Free PMC article. Review.
-
Syncytia formation affects the yield and cytotoxicity of an adenovirus expressing a fusogenic glycoprotein at a late stage of replication.Gene Ther. 2008 Sep;15(17):1240-5. doi: 10.1038/gt.2008.94. Epub 2008 May 29. Gene Ther. 2008. PMID: 18509378 Free PMC article.
-
In situ tumor vaccination with adenovirus vectors encoding measles virus fusogenic membrane proteins and cytokines.World J Gastroenterol. 2007 Jun 14;13(22):3063-70. doi: 10.3748/wjg.v13.i22.3063. World J Gastroenterol. 2007. PMID: 17589921 Free PMC article.
References
-
- Ahmed, A., D. Jevremovic, K. Suzuki, T. Kottke, J. Thompson, S. Emery, K. Harrington, A. Bateman, and R. Vile. 2003. Intratumoral expression of a fusogenic membrane glycoprotein enhances the efficacy of replicating adenovirus therapy. Gene Ther. 10:1663-1671. - PubMed
-
- Alemany, R., C. Balague, and D. T. Curiel. 2000. Replicative adenoviruses for cancer therapy. Nat. Biotechnol. 18:723-727. - PubMed
-
- Bateman, A., F. Bullough, S. Murphy, L. Emiliusen, D. Lavillette, F. L. Cosset, R. Cattaneo, S. J. Russell, and R. G. Vile. 2000. Fusogenic membrane glycoproteins as a novel class of genes for the local and immune-mediated control of tumor growth. Cancer Res. 60:1492-1497. - PubMed
-
- Bischoff, J. R., D. H. Kirn, A. Williams, C. Heise, S. Horn, M. Muna, L. Ng, J. A. Nye, A. Sampson-Johannes, A. Fattaey, and F. McCormick. 1996. An adenovirus mutant that replicates selectively in p53-deficient human tumor cells. Science 274:373-376. - PubMed
-
- Bucheit, A. D., S. Kumar, D. M. Grote, Y. Lin, V. von Messling, R. B. Cattaneo, and A. K. Fielding. 2003. An oncolytic measles virus engineered to enter cells through the CD20 antigen. Mol. Ther. 7:62-72. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

