Role of Gas6 receptors in platelet signaling during thrombus stabilization and implications for antithrombotic therapy
- PMID: 15650770
- PMCID: PMC544035
- DOI: 10.1172/JCI22079
Role of Gas6 receptors in platelet signaling during thrombus stabilization and implications for antithrombotic therapy
Abstract
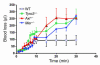
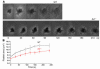
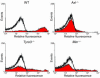
Mechanisms regulating thrombus stabilization remain largely unknown. Here, we report that loss of any 1 of the Gas6 receptors (Gas6-Rs), i.e., Tyro3, Axl, or Mer, or delivery of a soluble extracellular domain of Axl that traps Gas6 protects mice against life-threatening thrombosis. Loss of a Gas6-R does not prevent initial platelet aggregation but impairs subsequent stabilization of platelet aggregates, at least in part by reducing "outside-in" signaling and platelet granule secretion. Gas6, through its receptors, activates PI3K and Akt and stimulates tyrosine phosphorylation of the beta3 integrin, thereby amplifying outside-in signaling via alphaIIbbeta3. Blocking the Gas6-R-alphaIIbbeta3 integrin cross-talk might be a novel approach to the reduction of thrombosis.
Figures









References
-
- Prevost N, Woulfe D, Tognolini M, Brass LF. Contact-dependent signaling during the late events of platelet activation. J. Thromb. Haemost. 2003;1:1613–1627. - PubMed
-
- Law DA, et al. Integrin cytoplasmic tyrosine motif is required for outside-in alphaIIbbeta3 signalling and platelet function. Nature. 1999;401:808–811. - PubMed
-
- Phillips DR, Prasad KS, Manganello J, Bao M, Nannizzi-Alaimo L. Integrin tyrosine phosphorylation in platelet signaling. Curr. Opin. Cell Biol. 2001;13:546–554. - PubMed
-
- Pasquet JM, Noury M, Nurden AT. Evidence that the platelet integrin alphaIIb beta3 is regulated by the integrin-linked kinase, ILK, in a PI3-kinase dependent pathway. Thromb. Haemost. 2002;88:115–122. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

