Chronic lymphocytic leukemia B cells contain anomalous Lyn tyrosine kinase, a putative contribution to defective apoptosis
- PMID: 15650771
- PMCID: PMC544036
- DOI: 10.1172/JCI22094
Chronic lymphocytic leukemia B cells contain anomalous Lyn tyrosine kinase, a putative contribution to defective apoptosis
Abstract
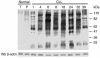
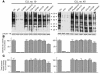
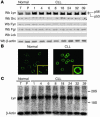
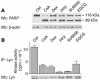
B cell chronic lymphocytic leukemia (B-CLL) is a neoplastic disorder characterized by accumulation of B lymphocytes due to uncontrolled growth and resistance to apoptosis. Analysis of B cells freshly isolated from 40 patients with chronic lymphocytic leukemia demonstrated that the Src kinase Lyn, the switch molecule that couples the B cell receptor to downstream signaling, displays anomalous properties. Lyn is remarkably overexpressed at the protein level in leukemic cells as compared with normal B lymphocytes, with a substantial aliquot of the kinase anomalously present in the cytosol. Whereas in normal B lymphocytes Lyn activation is dependent on B cell-receptor stimulation, in resting malignant cells, the constitutive activity of the kinase accounts for high basal protein tyrosine phosphorylation and low responsiveness to IgM ligation. Addition of the Lyn inhibitors PP2 and SU6656 to leukemic cell cultures restores cell apoptosis, and treatment of malignant cells with drugs that induce cell apoptosis decreases both activity and amount of the tyrosine kinase. These findings suggest a direct correlation between high basal Lyn activity and defects in the induction of apoptosis in leukemic cells. They also support a critical role for Lyn in B-CLL pathogenesis and identify this tyrosine kinase as a potential therapeutic target.
Figures



Similar articles
-
Valproic acid induces apoptosis in chronic lymphocytic leukemia cells through activation of the death receptor pathway and potentiates TRAIL response.Exp Hematol. 2007 Oct;35(10):1527-37. doi: 10.1016/j.exphem.2007.06.014. Epub 2007 Aug 13. Exp Hematol. 2007. PMID: 17697742
-
Matrix metalloproteinase-9 promotes chronic lymphocytic leukemia b cell survival through its hemopexin domain.Cancer Cell. 2010 Feb 17;17(2):160-72. doi: 10.1016/j.ccr.2009.12.044. Cancer Cell. 2010. PMID: 20159608
-
Analysis of migratory and prosurvival pathways induced by the homeostatic chemokines CCL19 and CCL21 in B-cell chronic lymphocytic leukemia.Exp Hematol. 2010 Sep;38(9):756-64, 764.e1-4. doi: 10.1016/j.exphem.2010.05.003. Epub 2010 May 19. Exp Hematol. 2010. PMID: 20488224
-
B-cell receptor signalling and its crosstalk with other pathways in normal and malignant cells.Eur J Haematol. 2015 Mar;94(3):193-205. doi: 10.1111/ejh.12427. Epub 2014 Sep 13. Eur J Haematol. 2015. PMID: 25080849 Review.
-
Association of Src-family kinase Lyn with B-cell antigen receptor.Immunol Rev. 1993 Apr;132:187-206. doi: 10.1111/j.1600-065x.1993.tb00843.x. Immunol Rev. 1993. PMID: 8349296 Review.
Cited by
-
Protein kinases: emerging therapeutic targets in chronic lymphocytic leukemia.Expert Opin Investig Drugs. 2012 Apr;21(4):409-23. doi: 10.1517/13543784.2012.668526. Epub 2012 Mar 9. Expert Opin Investig Drugs. 2012. PMID: 22409342 Free PMC article. Review.
-
Regulation of mitochondrial functions by protein phosphorylation and dephosphorylation.Cell Biosci. 2016 Apr 14;6:25. doi: 10.1186/s13578-016-0089-3. eCollection 2016. Cell Biosci. 2016. PMID: 27087918 Free PMC article. Review.
-
Novel Agents and Emerging Strategies for Targeting the B-Cell Receptor Pathway in CLL.Mediterr J Hematol Infect Dis. 2012;4(1):e2012067. doi: 10.4084/MJHID.2012.067. Epub 2012 Oct 9. Mediterr J Hematol Infect Dis. 2012. PMID: 23170196 Free PMC article.
-
Caspase-8 and Tyrosine Kinases: A Dangerous Liaison in Cancer.Cancers (Basel). 2023 Jun 21;15(13):3271. doi: 10.3390/cancers15133271. Cancers (Basel). 2023. PMID: 37444381 Free PMC article. Review.
-
The Src and c-Kit kinase inhibitor dasatinib enhances p53-mediated targeting of human acute myeloid leukemia stem cells by chemotherapeutic agents.Blood. 2013 Sep 12;122(11):1900-13. doi: 10.1182/blood-2012-11-466425. Epub 2013 Jul 29. Blood. 2013. PMID: 23896410 Free PMC article.
References
-
- Cheson BD, et al. National Cancer Institute-sponsored Working Group guidelines for chronic lymphocytic leukemia: revised guidelines for diagnosis and treatment. Blood. 1996;87:4990–4997. - PubMed
-
- Harris NL, et al. A revised European-American classification of lymphoid neoplasms: a proposal from the international lymphoma study group. Blood. 1994;84:1361–1392. - PubMed
-
- Caligaris-Cappio F, Ferrarini M. B cells and their fate in health and disease. Immunol. Today. 1996;17:206–208. - PubMed
-
- Hamblin TJ, Oscier DG. Chronic lymphocytic leukaemia: the nature of the leukaemic cell. Blood Rev. 1997;11:119–128. - PubMed
-
- Osterborg A, Mellstedt H, Keating M. Clinical effects of alemtuzumab (Campath-1H) in B-cell chronic lymphocytic leukemia. Med. Oncol. 2002;19(Suppl.):S21–S26. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

