MyD88-dependent induction of allergic Th2 responses to intranasal antigen
- PMID: 15650773
- PMCID: PMC544038
- DOI: 10.1172/JCI22462
MyD88-dependent induction of allergic Th2 responses to intranasal antigen
Abstract
MyD88 is a common Toll-like receptor (TLR) adaptor molecule found to be essential for induction of adaptive Th1 immunity. Conversely, innate control of adaptive Th2 immunity has been shown to occur in a MyD88-independent manner. In this study, we show that MyD88 is an essential innate component in the induction of TLR4-dependent Th2 responses to intranasal antigen; thus we demonstrate what we believe to be a novel role for MyD88 in pulmonary Th2 immunity. Induction of the MyD88-independent type I IFN response to LPS is defective in the pulmonary environment. Moreover, in the absence of MyD88, LPS-induced upregulation of costimulatory molecule expression on pulmonary DCs is defective, in contrast to what has been observed with bone marrow-derived DCs (BMDCs). Reconstitution of Th2 responses occurs upon adoptive pulmonary transfer of activated BMDCs to MyD88-deficient recipients. Furthermore, the dependence of Th2 responses on MyD88 is governed by the initial route of antigen exposure; this demonstrates what we believe are novel site-specific innate mechanisms for control of adaptive Th2 immunity.
Figures


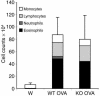
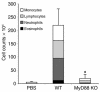

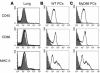
References
-
- Constant SL, Bottomly K. Induction of Th1 and Th2 CD4+ T cell responses: the alternative approaches. Annu. Rev. Immunol. 1997;15:297–322. - PubMed
-
- Eisenbarth SC, Piggott DA, Bottomly K. The master regulators of allergic inflammation: dendritic cells in Th2 sensitization. Curr. Opin. Immunol. 2003;15:620–626. - PubMed
-
- Lenschow DJ, Walunas TL, Bluestone JA. CD28/B7 system of T cell costimulation. Annu. Rev. Immunol. 1996;14:233–258. - PubMed
-
- Sharpe AH, Freeman GJ. The B7-CD28 superfamily. Nat. Rev. Immunol. 2002;2:116–126. - PubMed
-
- Janeway CA, Jr, Medzhitov R. Innate immune recognition. Annu. Rev. Immunol. 2002;20:197–216. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

