Membrane protein crystallization in amphiphile phases: practical and theoretical considerations
- PMID: 15652249
- PMCID: PMC2748814
- DOI: 10.1016/j.pbiomolbio.2004.07.006
Membrane protein crystallization in amphiphile phases: practical and theoretical considerations
Abstract
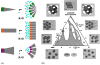
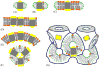
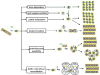
Integral membrane proteins are amphiphilic molecules. In order to enable chromatographic purification and crystallization, a complementary amphiphilic microenvironment must be created and maintained. Various types of amphiphilic phases have been employed in crystallizations and intricate amphiphilic microenvironmental structures have resulted from these and are found inside membrane protein crystals. In this review the process of crystallization is put into the context of amphiphile phase transitions. Finally, practical factors are considered and a pragmatic way is suggested to pursue membrane protein crystallization trials.
Figures

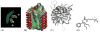


Similar articles
-
Widening the protein crystallization bottleneck.Nat Methods. 2006 Dec;3(12):961. doi: 10.1038/nmeth1206-961. Nat Methods. 2006. PMID: 17190005
-
Protein aggregation in silico.Trends Biotechnol. 2007 Jun;25(6):254-61. doi: 10.1016/j.tibtech.2007.03.011. Epub 2007 Apr 12. Trends Biotechnol. 2007. PMID: 17433843 Free PMC article. Review.
-
A model for enhanced nucleation of protein crystals on a fractal porous substrate.Biophys J. 2006 Nov 15;91(10):3857-63. doi: 10.1529/biophysj.106.082545. Epub 2006 Aug 18. Biophys J. 2006. PMID: 16920829 Free PMC article.
-
Pattern formation and fluctuation-induced transitions in protein crystallization.J Chem Phys. 2004 Apr 22;120(16):7708-19. doi: 10.1063/1.1687339. J Chem Phys. 2004. PMID: 15267682
-
Protein crystallization: from purified protein to diffraction-quality crystal.Nat Methods. 2008 Feb;5(2):147-53. doi: 10.1038/nmeth.f.203. Nat Methods. 2008. PMID: 18235435 Review.
Cited by
-
Frequencies of hydrophobic and hydrophilic runs and alternations in proteins of known structure.Protein Sci. 2006 Jan;15(1):102-12. doi: 10.1110/ps.051741806. Protein Sci. 2006. PMID: 16373477 Free PMC article.
-
Orientation and motion of tryptophan interfacial anchors in membrane-spanning peptides.Biochemistry. 2007 Jun 26;46(25):7514-24. doi: 10.1021/bi700082v. Epub 2007 May 27. Biochemistry. 2007. PMID: 17530863 Free PMC article.
-
The Fluidity of Phosphocholine and Maltoside Micelles and the Effect of CHAPS.Biophys J. 2019 May 7;116(9):1682-1691. doi: 10.1016/j.bpj.2019.03.019. Epub 2019 Mar 30. Biophys J. 2019. PMID: 31023535 Free PMC article.
-
Crystallographic characterization of N-oxide tripod amphiphiles.J Am Chem Soc. 2010 Feb 17;132(6):1953-9. doi: 10.1021/ja9085148. J Am Chem Soc. 2010. PMID: 20095541 Free PMC article.
-
Tripod Amphiphiles for Membrane Protein Manipulation.Mol Biosyst. 2010;6:89-94. doi: 10.1039/b915162c. Mol Biosyst. 2010. PMID: 23814603 Free PMC article.
References
-
- Alexandridis P, Olsson U, Lindman B. A record nine different phases (four cubic, two hexagonal, and one lamellar lyotropic liquid crystalline and two micellar solutions) in a ternary isothermal system of an amphiphilic block copolymer and selective solvents (water and oil) Langmuir. 1998;14:2627–2638.
-
- Belrhali H, Nollert P, Royant A, Menzel C, Rosenbusch J, Landau EM, Pebay-Peyroula E. Protein, lipid and water organization in bacteriorhodopsin crystals: a molecular view of the purple membrane at 1.9 angstrom resolution. Structure. 1999;7:909–917. - PubMed
-
- Buchanan SK. β-Barrel proteins in bacterial outer membranes: structure, function and refolding. Curr Opin Struct Biol. 1999;9:455–461. - PubMed
-
- Caffrey M. Membrane protein crystallization. J Struct Biol. 2003;142:108–132. - PubMed
-
- Cartailler J-P, Luecke H. X-ray crystallographic analysis of lipid-protein interactions in the bacteriorhodopsin purple membrane. Ann Rev Biophys Biomol Struct. 2003;32:285–310. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

