Membrane protein crystallization in amphiphile phases: practical and theoretical considerations
- PMID: 15652249
- PMCID: PMC2748814
- DOI: 10.1016/j.pbiomolbio.2004.07.006
Membrane protein crystallization in amphiphile phases: practical and theoretical considerations
Abstract
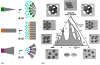
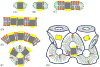
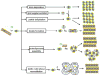
Integral membrane proteins are amphiphilic molecules. In order to enable chromatographic purification and crystallization, a complementary amphiphilic microenvironment must be created and maintained. Various types of amphiphilic phases have been employed in crystallizations and intricate amphiphilic microenvironmental structures have resulted from these and are found inside membrane protein crystals. In this review the process of crystallization is put into the context of amphiphile phase transitions. Finally, practical factors are considered and a pragmatic way is suggested to pursue membrane protein crystallization trials.
Figures

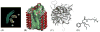


References
-
- Alexandridis P, Olsson U, Lindman B. A record nine different phases (four cubic, two hexagonal, and one lamellar lyotropic liquid crystalline and two micellar solutions) in a ternary isothermal system of an amphiphilic block copolymer and selective solvents (water and oil) Langmuir. 1998;14:2627–2638.
-
- Belrhali H, Nollert P, Royant A, Menzel C, Rosenbusch J, Landau EM, Pebay-Peyroula E. Protein, lipid and water organization in bacteriorhodopsin crystals: a molecular view of the purple membrane at 1.9 angstrom resolution. Structure. 1999;7:909–917. - PubMed
-
- Buchanan SK. β-Barrel proteins in bacterial outer membranes: structure, function and refolding. Curr Opin Struct Biol. 1999;9:455–461. - PubMed
-
- Caffrey M. Membrane protein crystallization. J Struct Biol. 2003;142:108–132. - PubMed
-
- Cartailler J-P, Luecke H. X-ray crystallographic analysis of lipid-protein interactions in the bacteriorhodopsin purple membrane. Ann Rev Biophys Biomol Struct. 2003;32:285–310. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

