Requirement of mesodermal retinoic acid generated by Raldh2 for posterior neural transformation
- PMID: 15652703
- PMCID: PMC2826194
- DOI: 10.1016/j.mod.2004.10.008
Requirement of mesodermal retinoic acid generated by Raldh2 for posterior neural transformation
Abstract
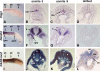
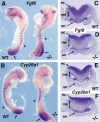
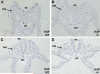
Studies in amphibian embryos have suggested that retinoic acid (RA) may function as a signal that stimulates posterior differentiation of the nervous system as postulated by the activation-transformation model for anteroposterior patterning of the nervous system. We have tested this hypothesis in retinaldehyde dehydrogenase-2 (Raldh2) null mutant mice lacking RA synthesis in the somitic mesoderm. Raldh2(-/-) embryos exhibited neural induction (activation) as evidenced by expression of Sox1 and Sox2 along the neural plate, but differentiation of spinal cord neuroectodermal progenitor cells (posterior transformation) did not occur as demonstrated by a loss of Pax6 and Olig2 expression along the posterior neural plate. Spinal cord differentiation in Raldh2(-/-) embryos was rescued by maternal RA administration, and during the rescue RA was found to act directly in the neuroectoderm but not the somitic mesoderm. RA generated by Raldh2 in the somitic mesoderm was found to normally travel as a signal throughout the mesoderm and neuroectoderm of the trunk and into tailbud neuroectoderm, but not into tailbud mesoderm. Raldh2(-/-) embryos also exhibited increased Fgf8 expression in the tailbud, and decreased cell proliferation in tailbud neuroectoderm. Our findings demonstrate that RA synthesized in the somitic mesoderm is necessary for posterior neural transformation in the mouse and that Raldh2 provides the only source of RA for posterior development. An important concept to emerge from our studies is that the somitic mesodermal RA signal acts in the neuroectoderm but not mesoderm to generate a spinal cord fate.
Figures


Similar articles
-
Retinoic acid regulation of the somitogenesis clock.Birth Defects Res C Embryo Today. 2007 Jun;81(2):84-92. doi: 10.1002/bdrc.20092. Birth Defects Res C Embryo Today. 2007. PMID: 17600781 Free PMC article. Review.
-
Novel retinoic acid generating activities in the neural tube and heart identified by conditional rescue of Raldh2 null mutant mice.Development. 2002 May;129(9):2271-82. doi: 10.1242/dev.129.9.2271. Development. 2002. PMID: 11959834 Free PMC article.
-
Retinoic-acid signalling in node ectoderm and posterior neural plate directs left-right patterning of somitic mesoderm.Nat Cell Biol. 2006 Mar;8(3):271-7. doi: 10.1038/ncb1374. Epub 2006 Feb 19. Nat Cell Biol. 2006. PMID: 16489341 Free PMC article.
-
Retinoic acid synthesis controlled by Raldh2 is required early for limb bud initiation and then later as a proximodistal signal during apical ectodermal ridge formation.J Biol Chem. 2004 Jun 18;279(25):26698-706. doi: 10.1074/jbc.M401920200. Epub 2004 Apr 6. J Biol Chem. 2004. PMID: 15069081
-
Retinoids and spinal cord development.J Neurobiol. 2006 Jun;66(7):726-38. doi: 10.1002/neu.20248. J Neurobiol. 2006. PMID: 16688770 Review.
Cited by
-
Retinoic acid upregulates ret and induces chain migration and population expansion in vagal neural crest cells to colonise the embryonic gut.PLoS One. 2013 May 22;8(5):e64077. doi: 10.1371/journal.pone.0064077. Print 2013. PLoS One. 2013. PMID: 23717535 Free PMC article.
-
Rewiring of the epigenome and chromatin architecture by exogenously induced retinoic acid signaling during zebrafish embryonic development.Nucleic Acids Res. 2024 Apr 24;52(7):3682-3701. doi: 10.1093/nar/gkae065. Nucleic Acids Res. 2024. PMID: 38321954 Free PMC article.
-
Knockdown of IKK1/2 promotes differentiation of mouse embryonic stem cells into neuroectoderm at the expense of mesoderm.Stem Cell Rev Rep. 2012 Dec;8(4):1098-108. doi: 10.1007/s12015-012-9402-7. Stem Cell Rev Rep. 2012. PMID: 22833419 Free PMC article.
-
Role of retinoic acid during forebrain development begins late when Raldh3 generates retinoic acid in the ventral subventricular zone.Dev Biol. 2007 Mar 15;303(2):601-10. doi: 10.1016/j.ydbio.2006.11.035. Epub 2006 Dec 2. Dev Biol. 2007. PMID: 17207476 Free PMC article.
-
Retinoic acid regulation of the somitogenesis clock.Birth Defects Res C Embryo Today. 2007 Jun;81(2):84-92. doi: 10.1002/bdrc.20092. Birth Defects Res C Embryo Today. 2007. PMID: 17600781 Free PMC article. Review.
References
-
- Bertrand N, Médevielle F, Pituello F. FGF signalling controls the timing of Pax6 activation in the neural tube. Development. 2000;127:4837–4843. - PubMed
-
- Del Corral RD, Breitkreuz DN, Storey KG. Onset of neuronal differentiation is regulated by paraxial mesoderm and requires attenuation of FGF signaling. Development. 2002;129:1681–1691. - PubMed
-
- Del Corral RD, Olivera-Martinez I, Goriely A, Gale E, Maden M, Storey K. Opposing FGF and retinoid pathways control ventral neural pattern, neuronal differentiation, and segmentation during body axis extension. Neuron. 2003;40:65–79. - PubMed
-
- Dobbs-McAuliffe B, Zhao QS, Linney E. Feedback mechanisms regulate retinoic acid production and degradation in the zebrafish embryo. Mech. Dev. 2004;121:339–350. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

