Cholinergic neurotransmission in the preBötzinger Complex modulates excitability of inspiratory neurons and regulates respiratory rhythm
- PMID: 15653001
- PMCID: PMC4342058
- DOI: 10.1016/j.neuroscience.2004.10.028
Cholinergic neurotransmission in the preBötzinger Complex modulates excitability of inspiratory neurons and regulates respiratory rhythm
Abstract
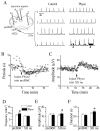
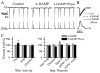
We investigated whether there is endogenous acetylcholine (ACh) release in the preBötzinger Complex (preBötC), a medullary region hypothesized to contain neurons generating respiratory rhythm, and how endogenous ACh modulates preBötCneuronal function and regulates respiratory pattern. Using a medullary slice preparation from neonatal rat, we recorded spontaneous respiratory-related rhythm from the hypoglossal nerve roots (XIIn) and patch-clamped preBötC inspiratory neurons. Unilateral microinjection of physostigmine, an acetylcholinesterase inhibitor, into the preBötC increased the frequency of respiratory-related rhythmic activity from XIIn to 116+/-13% (mean+/-S.D.) of control. Ipsilateral physostigmine injection into the hypoglossal nucleus (XII nucleus) induced tonic activity, increased the amplitude and duration of the integrated inspiratory bursts of XIIn to 122+/-17% and 117+/-22% of control respectively; but did not alter frequency. In preBötC inspiratory neurons, bath application of physostigmine (10 microM) induced an inward current of 6.3+/-10.6 pA, increased the membrane noise, decreased the amplitude of phasic inspiratory drive current to 79+/-16% of control, increased the frequency of spontaneous excitatory postsynaptic currents to 163+/-103% and decreased the whole cell input resistance to 73+/-22% of control without affecting the threshold for generation of action potentials. Bath application of physostigmine concurrently induced tonic activity, increased the frequency, amplitude and duration of inspiratory bursts of XIIn motor output. Bath application of 4-diphenylacetoxy-N-methylpiperidine methiodide (4-DAMP, 2 microM), a M3 muscarinic acetylcholine receptor (mAChR) selective antagonist, increased the input resistance of preBötC inspiratory neurons to 116+/-9% of control and blocked all of the effects of physostigmine except for the increase in respiratory frequency. Dihydro-beta-erythroidine (DH-beta-E; 0.2 microM), an alpha4beta2 nicotinic receptor (nAChR) selective antagonist, blocked all the effects of physostigmine except for the increase in inspiratory burst amplitude. In the presence of both 4-DAMP and DH-beta-E, physostigmine induced opposite effects, i.e. a decrease in frequency and amplitude of XIIn rhythmic activity. These results suggest that there is cholinergic neurotransmission in the preBötC which regulates respiratory frequency, and in XII nucleus which regulates tonic activity, and the amplitude and duration of inspiratory bursts of XIIn in neonatal rats. Physiologically relevant levels of ACh release, via mAChRs antagonized by 4-DAMP and nAChRs antagonized by DH-beta-E, modulate the excitability of inspiratory neurons and excitatory neurotransmission in the preBötC, consequently regulating respiratory rhythm.
Figures
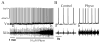



Similar articles
-
Mechanisms underlying regulation of respiratory pattern by nicotine in preBötzinger complex.J Neurophysiol. 2001 Jun;85(6):2461-7. doi: 10.1152/jn.2001.85.6.2461. J Neurophysiol. 2001. PMID: 11387392 Free PMC article.
-
Pharmacology of nicotinic receptors in preBötzinger complex that mediate modulation of respiratory pattern.J Neurophysiol. 2002 Oct;88(4):1851-8. doi: 10.1152/jn.2002.88.4.1851. J Neurophysiol. 2002. PMID: 12364511 Free PMC article.
-
Acetylcholine modulates respiratory pattern: effects mediated by M3-like receptors in preBötzinger complex inspiratory neurons.J Neurophysiol. 2000 Mar;83(3):1243-52. doi: 10.1152/jn.2000.83.3.1243. J Neurophysiol. 2000. PMID: 10712452 Free PMC article.
-
Central cholinergic regulation of respiration: nicotinic receptors.Acta Pharmacol Sin. 2009 Jun;30(6):761-70. doi: 10.1038/aps.2009.88. Acta Pharmacol Sin. 2009. PMID: 19498418 Free PMC article. Review.
-
Rubral modulation of breathing.Exp Physiol. 2019 Nov;104(11):1595-1604. doi: 10.1113/EP087720. Epub 2019 Oct 9. Exp Physiol. 2019. Retraction in: Exp Physiol. 2020 Nov;105(11):1971. doi: 10.1113/EP089094. PMID: 31408227 Retracted. Review.
Cited by
-
Combined unilateral blockade of cholinergic, peptidergic, and serotonergic receptors in the ventral respiratory column does not affect breathing in awake or sleeping goats.J Appl Physiol (1985). 2015 Aug 1;119(3):308-20. doi: 10.1152/japplphysiol.00145.2015. Epub 2015 May 28. J Appl Physiol (1985). 2015. PMID: 26023224 Free PMC article.
-
Cyclothiazide-induced persistent increase in respiratory-related activity in vitro.J Physiol. 2012 Oct 1;590(19):4897-915. doi: 10.1113/jphysiol.2012.232421. Epub 2012 Jul 2. J Physiol. 2012. PMID: 22753547 Free PMC article.
-
Long lasting effects of perinatal exposure to the Chlorpyrifos pesticide on sleep, breathing, and neuroinflammation in adult mice.PLoS One. 2025 Aug 1;20(8):e0328581. doi: 10.1371/journal.pone.0328581. eCollection 2025. PLoS One. 2025. PMID: 40748870 Free PMC article.
-
Influence of prenatal nicotine exposure on development of the ventilatory response to hypoxia and hypercapnia in neonatal rats.J Appl Physiol (1985). 2010 Jul;109(1):149-58. doi: 10.1152/japplphysiol.01036.2009. Epub 2010 Apr 29. J Appl Physiol (1985). 2010. PMID: 20431025 Free PMC article.
-
Mechanisms of Organophosphate Toxicity and the Role of Acetylcholinesterase Inhibition.Toxics. 2023 Oct 18;11(10):866. doi: 10.3390/toxics11100866. Toxics. 2023. PMID: 37888716 Free PMC article. Review.
References
-
- Adams GK, Yamamura HI, O’Leary JF. Recovery of central respiratory function following anticholinesterase intoxication. Eur J Pharmacol. 1976;38:101–112. - PubMed
-
- Akaike N, Moorhouse AJ. Techniques: applications of the nerve-bouton preparation in neuropharmacology. Trends Pharmacol Sci. 2003;24:44–47. - PubMed
-
- Aldes LD. Subcompartmental organization of the ventral (protrusor) compartment in the hypoglossal nucleus of the rat. J Comp Neurol. 1995;353:89–108. - PubMed
-
- Alkondon M, Albuquerque EX. Nicotinic acetylcholine receptor alpha7 and alpha4beta2 subtypes differentially control GABAergic input to CA1 neurons in rat hippocampus. J Neurophysiol. 2001;86:3043–3055. - PubMed
-
- Alkondon M, Braga MF, Pereira EF, Maelicke A, Albuquerque EX. alpha7 nicotinic acetylcholine receptors and modulation of gabaergic synaptic transmission in the hippocampus. Eur J Pharmacol. 2000;393:59–67. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

