On the importance of the phosphocholine methyl groups for sphingomyelin/cholesterol interactions in membranes: a study with ceramide phosphoethanolamine
- PMID: 15653729
- PMCID: PMC1305362
- DOI: 10.1529/biophysj.104.058149
On the importance of the phosphocholine methyl groups for sphingomyelin/cholesterol interactions in membranes: a study with ceramide phosphoethanolamine
Abstract
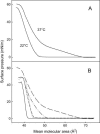
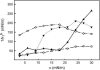
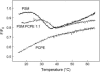
In this study, we have examined how the headgroup size and properties affect the membrane properties of sphingomyelin and interactions with cholesterol. We prepared N-palmitoyl ceramide phosphoethanolamine (PCPE) and compared its membrane behavior with D-erythro-N-palmitoyl-sphingomyelin (PSM), both in monolayers and bilayers. The pure PCPE monolayer did not show a phase transition at 22 degrees C (in contrast to PSM), but displayed a much higher inverse isothermal compressibility as compared to the PSM monolayer, indicating stronger intermolecular interactions between PCPEs than between PSMs. At 37 degrees C the PCPE monolayer was more expanded (than at 22 degrees C) and displayed a rather poorly defined phase transition. When cholesterol was comixed into the monolayer, a condensing effect of cholesterol on the lateral packing of the lipids in the monolayer could be observed. The phase transition from an ordered to a disordered state in bilayer membranes was determined by diphenylhexatriene steady-state anisotropy. Whereas the PSM bilayer became disordered at 41 degrees C, the PCPE bilayer main transition occurred around 64 degrees C. The diphenylhexatriene steady-state anisotropy values were similar in both PCPE and PSM bilayers before and after the phase transition, suggesting that the order in the hydrophobic core in both bilayer types was rather similar. The emission from Laurdan was blue shifted in PCPE bilayers in the gel phase when compared to the emission spectra from PSM bilayers, and the blue-shifted component in PCPE bilayers was retained also after the phase transition, suggesting that Laurdan molecules sensed a more hydrophobic environment at the PCPE interface compared to the PSM interface both below and above the bilayer melting temperature. Whereas PSM was able to form sterol-enriched domains in dominantly fluid bilayers (as determined from cholestatrienol dequenching experiments), PCPE failed to form such domains, suggesting that the size and/or properties of the headgroup was important for stabilizing sphingolipid/sterol interaction. In conclusion, our study has highlighted how the headgroup in sphingomyelin affect its membrane properties and interactions with cholesterol.
Figures



Similar articles
-
Cholesterol's interactions with serine phospholipids - a comparison of N-palmitoyl ceramide phosphoserine with dipalmitoyl phosphatidylserine.Biochim Biophys Acta. 2013 Feb;1828(2):785-91. doi: 10.1016/j.bbamem.2012.11.009. Epub 2012 Nov 13. Biochim Biophys Acta. 2013. PMID: 23159809
-
N-cholesteryl sphingomyelin-A synthetic sphingolipid with unique membrane properties.Biochim Biophys Acta. 2011 Apr;1808(4):1054-62. doi: 10.1016/j.bbamem.2010.12.021. Epub 2010 Dec 29. Biochim Biophys Acta. 2011. PMID: 21194522
-
N- and O-methylation of sphingomyelin markedly affects its membrane properties and interactions with cholesterol.Biochim Biophys Acta. 2011 Apr;1808(4):1179-86. doi: 10.1016/j.bbamem.2011.01.009. Epub 2011 Jan 22. Biochim Biophys Acta. 2011. PMID: 21262197
-
Sphingolipids and the formation of sterol-enriched ordered membrane domains.Biochim Biophys Acta. 2006 Dec;1758(12):1945-56. doi: 10.1016/j.bbamem.2006.05.020. Epub 2006 Jun 3. Biochim Biophys Acta. 2006. PMID: 16901461 Review.
-
The importance of hydrogen bonding in sphingomyelin's membrane interactions with co-lipids.Biochim Biophys Acta. 2016 Feb;1858(2):304-10. doi: 10.1016/j.bbamem.2015.12.008. Epub 2015 Dec 4. Biochim Biophys Acta. 2016. PMID: 26656158 Review.
Cited by
-
N-nervonoylsphingomyelin (C24:1) prevents lateral heterogeneity in cholesterol-containing membranes.Biophys J. 2014 Jun 17;106(12):2606-16. doi: 10.1016/j.bpj.2014.04.054. Biophys J. 2014. PMID: 24940778 Free PMC article.
-
Molecular dynamics simulations of SOPS and sphingomyelin bilayers containing cholesterol.Biophys J. 2007 Feb 15;92(4):1284-95. doi: 10.1529/biophysj.106.096214. Epub 2006 Dec 1. Biophys J. 2007. PMID: 17142272 Free PMC article.
-
Characterization of the ternary mixture of sphingomyelin, POPC, and cholesterol: support for an inhomogeneous lipid distribution at high temperatures.Biophys J. 2008 Apr 1;94(7):2680-90. doi: 10.1529/biophysj.107.112904. Epub 2008 Jan 4. Biophys J. 2008. PMID: 18178660 Free PMC article.
-
Sphingomyelin synthase-related protein SMSr controls ceramide homeostasis in the ER.J Cell Biol. 2009 Jun 15;185(6):1013-27. doi: 10.1083/jcb.200903152. Epub 2009 Jun 8. J Cell Biol. 2009. PMID: 19506037 Free PMC article.
-
The effect of cholesterol on the long-range network of interactions established among sea anemone Sticholysin II residues at the water-membrane interface.Mar Drugs. 2015 Mar 25;13(4):1647-65. doi: 10.3390/md13041647. Mar Drugs. 2015. PMID: 25815890 Free PMC article.
References
-
- Ahmed, S. N., D. A. Brown, and E. London. 1997. On the origin of sphingolipid/cholesterol-rich detergent soluble cell membranes: physiological concentrations of cholesterol and sphingolipid induce formation of a detergent insoluble, liquid ordered lipid phase in model membranes. Biochemistry. 36:10944–10953. - PubMed
-
- Bach, D., and E. Wachtel. 2003. Phospholipid/cholesterol model membranes: formation of cholesterol crystallites. Biochim. Biophys. Acta. 1610:187–197. - PubMed
-
- Bagatolli, L. A., T. Parasassi, G. D. Fidelio, and E. Gratton. 1999. A model for the interaction of 6-lauroyl-2-(N,N-dimethylamino)naphthalene with lipid environments: implication for spectral properties. Photochem. Photobiol. 70:557–564. - PubMed
-
- Bittman, R., C. R. Kasireddy, P. Mattjus, and J. P. Slotte. 1994. Interaction of cholesterol with sphingomyelin in monolayers and vesicles. Biochemistry. 33:11776–11781. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

