Nano- to microscale dynamics of P-selectin detachment from leukocyte interfaces. II. Tether flow terminated by P-selectin dissociation from PSGL-1
- PMID: 15653735
- PMCID: PMC1305279
- DOI: 10.1529/biophysj.104.051706
Nano- to microscale dynamics of P-selectin detachment from leukocyte interfaces. II. Tether flow terminated by P-selectin dissociation from PSGL-1
Abstract
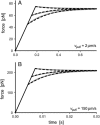
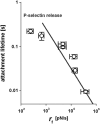
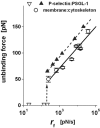
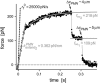
We have used a biomembrane force probe decorated with P-selectin to form point attachments with PSGL-1 receptors on a human neutrophil (PMN) in a calcium-containing medium and then to quantify the forces experienced by the attachment during retraction of the PMN at fixed speed. From first touch to final detachment, the typical force history exhibited the following sequence of events: i), an initial linear-elastic displacement of the PMN surface, ii), an abrupt crossover to viscoplastic flow that signaled membrane separation from the interior cytoskeleton and the beginning of a membrane tether, and iii), the final detachment from the probe tip most often by one precipitous step of P-selectin:PSGL-1 dissociation. Analyzing the initial elastic response and membrane unbinding from the cytoskeleton in our companion article I, we focus in this article on the regime of tether extrusion that nearly always occurred before release of the extracellular adhesion bond at pulling speeds > or =1 microm/s. The force during tether growth appeared to approach a plateau at long times. Examined over a large range of pulling speeds up to 150 microm/s, the plateau force exhibited a significant shear thinning as indicated by a weak power-law dependence on pulling speed, f(infinity) = 60 pN(nu(pull)/microm/s)(0.25). Using this shear-thinning response to describe the viscous element in a nonlinear Maxwell-like fluid model, we show that a weak serial-elastic component with a stiffness of approximately 0.07 pN/nm provides good agreement with the time course of the tether force approach to the plateau under constant pulling speed.
Figures






Similar articles
-
Nano- to microscale dynamics of P-selectin detachment from leukocyte interfaces. I. Membrane separation from the cytoskeleton.Biophys J. 2005 Mar;88(3):2288-98. doi: 10.1529/biophysj.104.051698. Epub 2005 Jan 14. Biophys J. 2005. PMID: 15653718 Free PMC article.
-
Nano-to-micro scale dynamics of P-selectin detachment from leukocyte interfaces. III. Numerical simulation of tethering under flow.Biophys J. 2005 Mar;88(3):1676-83. doi: 10.1529/biophysj.104.051805. Epub 2004 Dec 1. Biophys J. 2005. PMID: 15574709 Free PMC article.
-
Neutrophil-bead collision assay: pharmacologically induced changes in membrane mechanics regulate the PSGL-1/P-selectin adhesion lifetime.Biophys J. 2005 Nov;89(5):3603-14. doi: 10.1529/biophysj.105.066134. Epub 2005 Aug 12. Biophys J. 2005. PMID: 16100264 Free PMC article.
-
Mechanisms for flow-enhanced cell adhesion.Ann Biomed Eng. 2008 Apr;36(4):604-21. doi: 10.1007/s10439-008-9464-5. Epub 2008 Feb 26. Ann Biomed Eng. 2008. PMID: 18299992 Free PMC article. Review.
-
Receptor-ligand binding: 'catch' bonds finally caught.Curr Biol. 2003 Aug 5;13(15):R611-3. doi: 10.1016/s0960-9822(03)00529-3. Curr Biol. 2003. PMID: 12906816 Review.
Cited by
-
Effects of plasma membrane cholesterol level and cytoskeleton F-actin on cell protrusion mechanics.PLoS One. 2013;8(2):e57147. doi: 10.1371/journal.pone.0057147. Epub 2013 Feb 22. PLoS One. 2013. PMID: 23451167 Free PMC article.
-
Effect of viscoelasticity on the analysis of single-molecule force spectroscopy on live cells.Biophys J. 2012 Jul 3;103(1):137-45. doi: 10.1016/j.bpj.2012.05.044. Biophys J. 2012. PMID: 22828340 Free PMC article.
-
Probing mechanical interaction of immune receptors and cytoskeleton by membrane nanotube extraction.Sci Rep. 2023 Sep 20;13(1):15652. doi: 10.1038/s41598-023-42599-9. Sci Rep. 2023. PMID: 37730849 Free PMC article.
-
Versatile horizontal force probe for mechanical tests on pipette-held cells, particles, and membrane capsules.Biophys J. 2009 Feb;96(3):1218-31. doi: 10.1016/j.bpj.2008.10.047. Biophys J. 2009. PMID: 19186156 Free PMC article.
-
Cell Membrane Tension Gradients, Membrane Flows, and Cellular Processes.Physiology (Bethesda). 2024 Jul 1;39(4):0. doi: 10.1152/physiol.00007.2024. Epub 2024 Mar 19. Physiology (Bethesda). 2024. PMID: 38501962 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

